Computer lab
Enzyme kinetics and characterization of reaction intermediates ÅKR 2004
The heme peroxidases utilize a heme cofactor and H2O2 (hydrogen peroxide) to create a radical on the substrate. A radical is an unpaired electron, and such species are usually highly reactive. Radicals are usually illustrated with the symbol . Substrate/product radicals will immediately react with each other and form stable products.
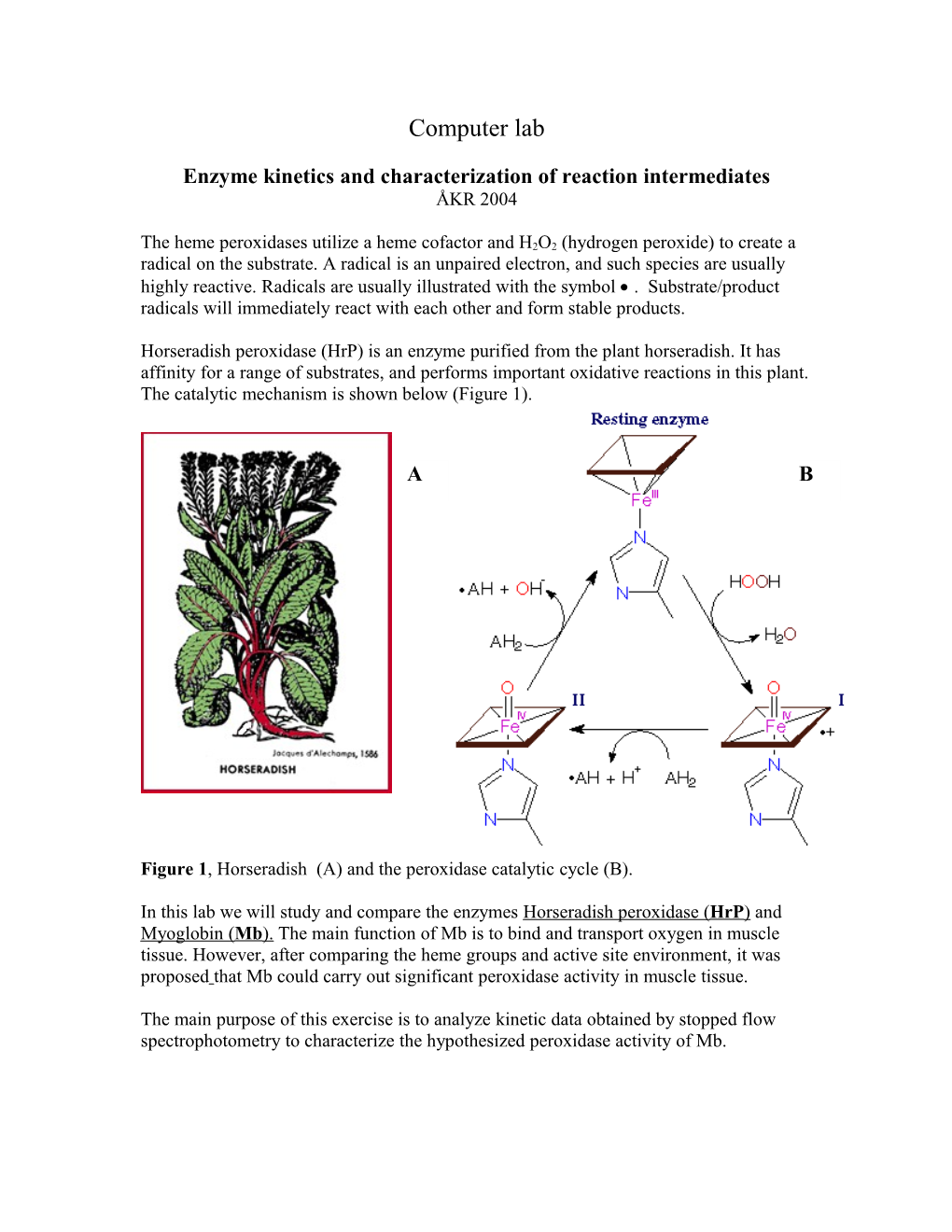
Horseradish peroxidase (HrP) is an enzyme purified from the plant horseradish. It has affinity for a range of substrates, and performs important oxidative reactions in this plant. The catalytic mechanism is shown below (Figure 1).
A B
Figure 1, Horseradish (A) and the peroxidase catalytic cycle (B).
In this lab we will study and compare the enzymes Horseradish peroxidase ( HrP ) and Myoglobin ( Mb ). The main function of Mb is to bind and transport oxygen in muscle tissue. However, after comparing the heme groups and active site environment, it was proposed that Mb could carry out significant peroxidase activity in muscle tissue.
The main purpose of this exercise is to analyze kinetic data obtained by stopped flow spectrophotometry to characterize the hypothesized peroxidase activity of Mb. Summary of experiments:
1) Determine the Vmax and kcat for HrP and Mb at pH = 6.5 and T = 20 C using different concentrations of the substrate ortho-phenylenediamine (OPD).
2) Investigate the rapid stopped flow kinetics (in ms time scale) for the reaction of enzyme and H2O2.
3) Discuss which properties that influence the catalytic efficiency.
NB! SAVE ALL FIGURES YOU MAKE DURING THE EXERCISES
Determination of kinetic constants for HrP and Mb using OPD as substrate
Ortho-phenylenediamine is an excellent substrate for HrP, and because a colorless substrate is converted to an orange-brown product, the reaction progress can easily be monitored by spectrophotometry.
We will use the Michaelis –Menten formalism to extract the Vmax parameter and subsequently calculate the turnover number, kcat, that describes the efficiency of the enzymes.
Description of Michaelis – Menten experiment:
1) Import data in OPJ files. 2) Determine initial rates from the absorption at 450 nm. 3) Make a Michaelis-Menten plot (initial rate versus [OPD]). 4) Fit data, calculate kcat and answer questions. Exercise 1
Make a plot from data showing the production of DAP and calculate the extinction coefficient of this product:
1) Open the file Epsilon determination.OPJ 2) Plot the spectra recorded at 3, 30, 55, and 60 seconds in the same graph.
At which wavelength is the product (DAP) absorption maximum found?
The last spectrum (60 s) was recorded when all substrate had been converted to product. The initial substrate concentration was 0.1 mM.
-1 -1 Calculate the extinction coefficient 450 (mM cm ) of the product DAP at 450 nm by using Beers law; A450 = 450 [DAP] 1 cm . Remember that 2 OPD DAP.
What is the extinction coefficient 450 of DAP? Why do we use the last spectrum to calculate extinction coefficient? Exercise 2
Determination of Vmax and kcat
Different concentrations of substrate have been added to Mb and HrP. Stopped flow data at 450 nm have been recorded and imported into Origin OPJ files. HRP_MM.OPJ and MB_MM.OPJ contains the HrP and Mb Michaelis –Menten data, respectively.
Data files HrP Mb [OPD] mM Worksheet name [OPD] mM Worksheet name 0.05 HRP0050 0.02 MB0020 0.1 HRP0100 0.03 MB0030 0.15 HRP0150 0.05 MB0050 0.2 HRP0200 0.15 MB0150 0.3 HRP0300 0.2 MB0200 0.5 HRP0500 0.3 MB0300 0.8 HRP0800 - - 1.2 HRP1200 - -
Find initial rates (v0) for all reactions
Open OPJ file for HrP or Mb. Plot all the curves in one graph using to see the effect of increasing substrate concentration. Include this graph in the report. Do not include all individual linear fittings. (Tips; plot the first worksheet and drag the other Y-data into the graph).
To determine the initial velocity (v0) for each substrate concentration, do a linear fit of the first 5-10 data points, it should be a relatively straight line:
Select the first 10 data points. Press to make a graph. Analyzis -> Fit linear (the result of the fit shows up in the box at the lower right corner of the screen, scroll and find the slope B).
V0 = B/ 0.5 * 450, DAP Calculate v0 for all data sets, and write them down. Michaelis- Menten plot
For each protein, create a worksheet with [OPD] in the X-column and v0 in the Y- column.
Plot each worksheet with and fit using the Advanced fitting tool.
Press: Analysis -> Non linear curve fit -> Advanced fitting tool.
A new dialog box appers:
Press: Function -> Select
Choose:
Category = Pharmacology
Function = OneSiteBind
This function resembles the Michaelis –Menten equation:
B = Vmax K = Km X = [OPD]
Press: Action -> Fit, Active dataset.
Choose all initial fitting parameters = 1 and press 100 Iter. If the fitting fails, use the horizontal asymptote of the plot as a approximation for B.
Write down the best fitting parameters.
The enzyme concentrations used are [HrP] = 1.0 *10-5 mM and [Mb] = 5.8 *10-3 mM
For both HrP and Mb, calculate kcat = Vmax / [Enzyme]
What does the kcat constants tell you about the of the reactions? How many times faster is HrP than Mb catalyzing the reaction 2 OPD DAP ? Exercise 3
Introduction
Rapid stopped flow kinetics detection of intermediates
The reaction cycle of HrP is shown in Figure 1. Two intermediates, compound I (A) and compound II (B), can be detected when the enzyme reacts with H2O2.
A B
In this experiment the ferric (Fe3+) form of the two enzymes HrP and Mb are allowed to react with hydrogen peroxide (H2O2) in absence of the substrate OPD. We want to study the reaction of H2O2 with the two enzymes HrP and Mb. Perhaps we can gain some insight that will explain the difference in catalytic efficiency between the two enzymes?
Soret () band
and bands
350 400 450 500 550 600 650 700 Wavelenght (nm) Figure 2. Visible spectrum of ferric (Fe3+) HrP
p p* p p* (, Low intensity) (, High intensity) p p* (, Low intensity) Charge Transfer Low intensity
350 400 450 500 550 600 650 700 Wavelength (nm)Wavelength
450 500 550 600 650 700 Wavelength
Figure 3. Visible spectrum of ferric (Fe3+) Mb
Experimental
The data files used in this exercise are stored in the catalogs “Rapid_kinetics_HrP.OPJ” and “Rapid_kinetics_Mb.OPJ”
1) Make a plot where you compare the “resting” ferric HrP with the following stopped flow data (2D-graph). The interesting region is 450 – 700 nm. 0 s (Hrpresting) 75 ms (HrP0075, first spectrum) 15 s (HrPH15, last spectrum) 2) Make similar plot for Mb 0 s (Mbresting) 5 ms (Mb0005, first spectrum) 500 ms (Mb05, last spectrum) A) From the plot for HrP at different times, make a table of the absorbance values at 496 nm, 543 nm, 578 nm and 650 nm at 0 s, 75 ms, and 15 s. Are there any peaks that first seem to increase/decrease in the first time interval (0 s – 75 ms, this is resting HrP to first rapid kinetics spectrum) and then increase/ decrease in the second time interval (75 ms – 15 s)?
Which spectrum corresponds to which intermediate in Figure 1? *
For each wavelength (496, 543, 578, and 650 nm), calculate first order rate constants k using: Analysis -> Fit exponential decay -> First order.
TIPS!! If the fitting is bad (e.g. t1 > 10 ), use the Advanced fitting tool, select the exponential category and use the function ExpDec1 with the parameter A1 < 0.
The rate constant k = 1/ t1 (the value for t1 can be found in the result box in the graph after fitting). A high rate constant indicates a fast reaction.
Insert the calculated rate constants in the table below.
First Order Rate Constants for HrP Wavlelength 496 nm 543 nm 578 nm 650 nm 75ms – 15s
B)
Repeat the procedure above for Mb, however, the interesting and corresponding wavelengths are now 503, 550, 588, and 640 nm. Skip the question above marked with “*” and answer this one instead: In the Mb + H2O2 reaction only one of the intermediates in Figure 1 is observed. Which one? (Hint: look for similar spectral features).
First Order Rate Constants for Mb Wavlelength 503 nm 550 nm 588 nm 640 nm 5 – 500 ms Both compound I and compound II are very reactive. Consider the results from the Michaelis- Menten experiments (calculation of the turnover number kcat) and the rapid stopped flow studies of reaction intermediates in the absence of substrate.
Compare HrP and Mb and consider: 1) How fast they react with H2O2. 2) The rate of product (DAP) formation.
What do you think determine the formation of the product DAP from OPD?
Considering the low substrate affinity of H2O2 activated Mb; do you think Mb can carry out significant peroxidase activity in human tissue?