Name______Concentration Worksheet
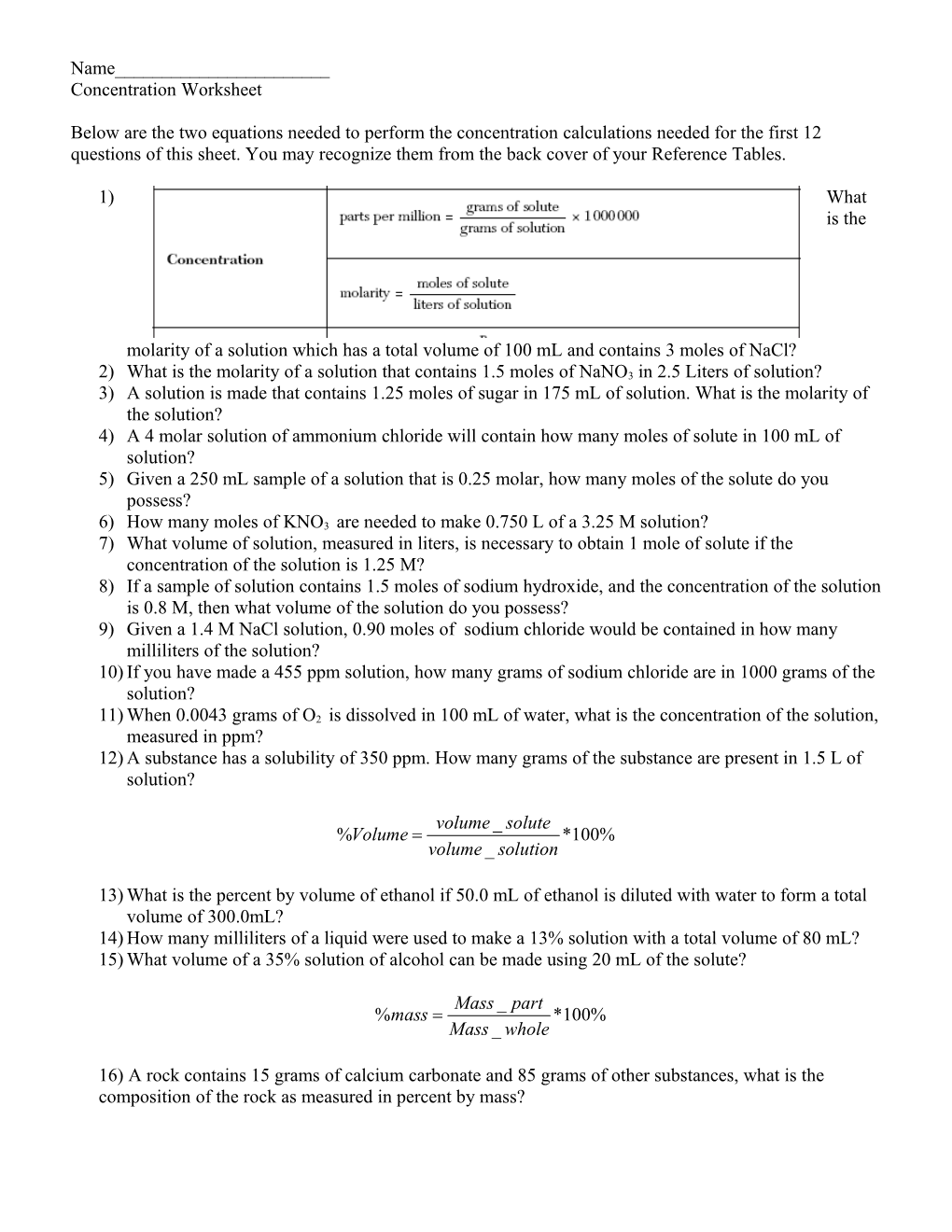
Below are the two equations needed to perform the concentration calculations needed for the first 12 questions of this sheet. You may recognize them from the back cover of your Reference Tables.
1) What is the
molarity of a solution which has a total volume of 100 mL and contains 3 moles of NaCl? 2) What is the molarity of a solution that contains 1.5 moles of NaNO3 in 2.5 Liters of solution? 3) A solution is made that contains 1.25 moles of sugar in 175 mL of solution. What is the molarity of the solution? 4) A 4 molar solution of ammonium chloride will contain how many moles of solute in 100 mL of solution? 5) Given a 250 mL sample of a solution that is 0.25 molar, how many moles of the solute do you possess? 6) How many moles of KNO3 are needed to make 0.750 L of a 3.25 M solution? 7) What volume of solution, measured in liters, is necessary to obtain 1 mole of solute if the concentration of the solution is 1.25 M? 8) If a sample of solution contains 1.5 moles of sodium hydroxide, and the concentration of the solution is 0.8 M, then what volume of the solution do you possess? 9) Given a 1.4 M NaCl solution, 0.90 moles of sodium chloride would be contained in how many milliliters of the solution? 10) If you have made a 455 ppm solution, how many grams of sodium chloride are in 1000 grams of the solution? 11) When 0.0043 grams of O2 is dissolved in 100 mL of water, what is the concentration of the solution, measured in ppm? 12) A substance has a solubility of 350 ppm. How many grams of the substance are present in 1.5 L of solution?
volume _ solute %Volume *100% volume _ solution
13) What is the percent by volume of ethanol if 50.0 mL of ethanol is diluted with water to form a total volume of 300.0mL? 14) How many milliliters of a liquid were used to make a 13% solution with a total volume of 80 mL? 15) What volume of a 35% solution of alcohol can be made using 20 mL of the solute?
Mass _ part %mass *100% Mass _ whole
16) A rock contains 15 grams of calcium carbonate and 85 grams of other substances, what is the composition of the rock as measured in percent by mass?