9.1 Carbon Compounds
Organic compounds contains C and H (often combined w/ O and N) – C-C bonds and C-H bonds There are millions of organic compounds = more than 90% of known compounds C has 4 valence e- lots of options for bonds 4 single bonds 2 single + 1 double 2 double 1 triple + 1 single
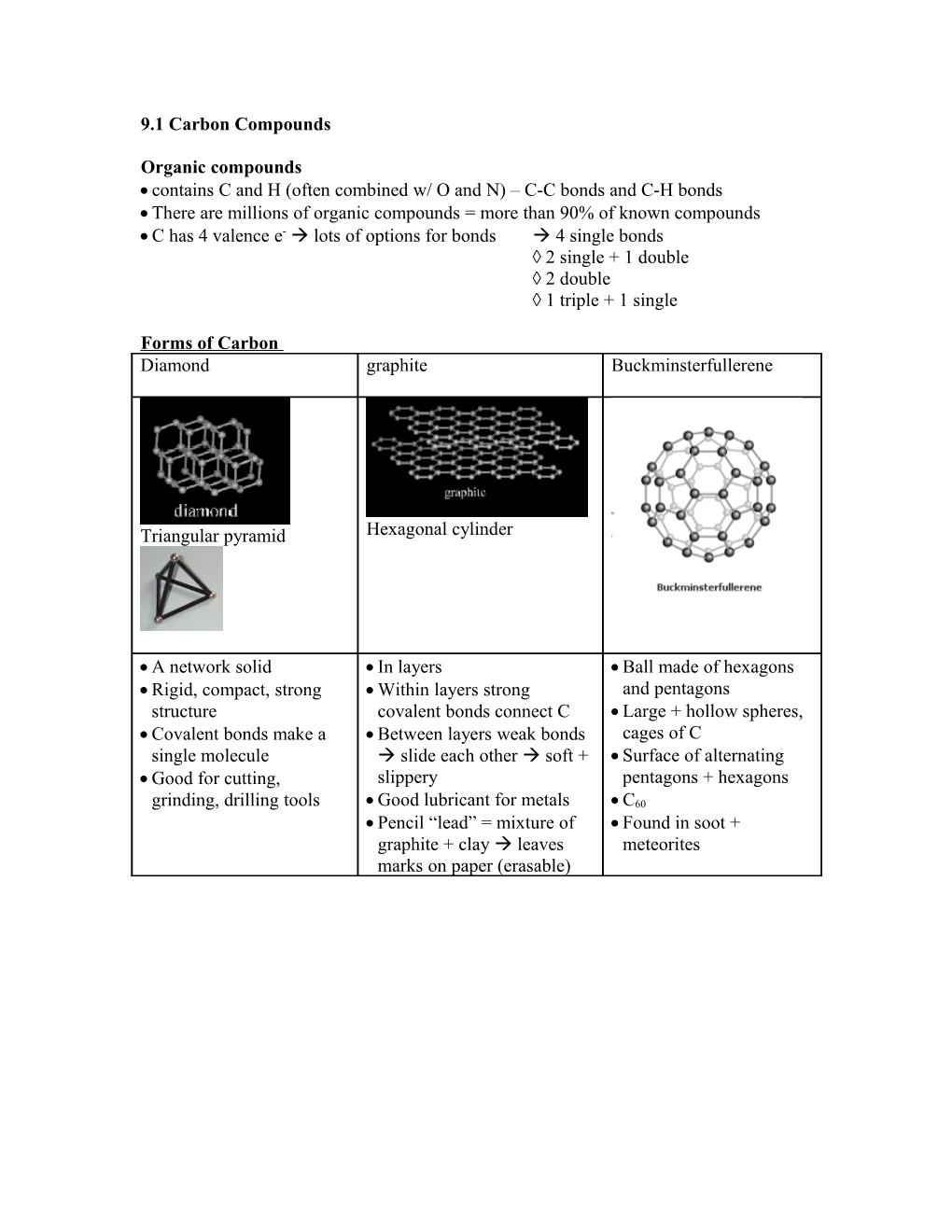
Forms of Carbon Diamond graphite Buckminsterfullerene
Triangular pyramid Hexagonal cylinder
A network solid In layers Ball made of hexagons Rigid, compact, strong Within layers strong and pentagons structure covalent bonds connect C Large + hollow spheres, Covalent bonds make a Between layers weak bonds cages of C single molecule slide each other soft + Surface of alternating Good for cutting, slippery pentagons + hexagons grinding, drilling tools Good lubricant for metals C60 Pencil “lead” = mixture of Found in soot + graphite + clay leaves meteorites marks on paper (erasable) Hydrocarbon =Organic compound made of H + C – properties determined by # of C + arrangement of atoms Saturated hydrocarbons Unsaturated hydrocarbons Alkanes – single bonds C-C (-ane) Alkenes – double bond C=C Alkynes –triple bond C≡C (-yne) CnHsn+2 (-ene) w/ 1 double bond CnH2n w/ 1 triple bond CnH2n-2 ethyne
propyne
1 – meth- 6 – hex- 2 – eth- 7 – Sept- 3 – prop- 8 – oct- 4 – but- 9 -non- 5 – pent- 10 -deca- Saturated hydrocarbons – only single bonds 1) straight chains - see above table in alkane column
Methane (CH4) in cow’s stomach can break down cellulose in grass Methane + Propane = gases
Pentane(C5H12) + octane (C8H18) = liquids More carbon atoms higher boiling points
2) branched chains - isomers of straight chains (isomer – same molecular formula, different structural formula)
isobutane (C4H10)
# of isomers increases when # of carbon of alkanes increases: ex) octane (C8H18) has 18 isomers – decane (C10H22) has 75 isomers Boiling pt of butane -0.5ºC; boiling pt of isobutene = -11.7ºC
3) Rings – C-C-C bonds only 2 H connected to each C = “cyclo-“
cyclobutane (C4H8) – 2 less H than C4H10
cyclopentane (C5H10) – 2 less H than C5H12 Unsaturated Hydrocarbons – alkenes, alkynes, aromatic hydrocarbons
Alkenes Alkynes Aromatic Hydrocarbons double bonds C=C Double bonds C≡C Ring structure (cyclical)
C2H4 controls the rate at C2H2 =ethyne = Strong aromas or odors which fruits (ex. acetylene alternating single and double Tomato) ripens; reacts Most reactive bonds but six bonds are identical chemically to produce hydrocarbon ex) C6H6 plastic (garbage bags, compounds milk jugs) acetylene + oxygen oxyacetylene torch (high temperature 3500ºC)
C6H6 (benzene)
Fossil Fuels - remains of animals + plants from millions of years ago turned into deposits of hydrocarbons Coal Natural gas petroleum Giant tree ferns + other Remains of marine remains of marine plants buried in swamps organisms organisms Mostly aromatic Main component = pumped from deep hydrcarbons methane (CH4) = beneath Earth’s surface High molar masses compound produced by composed of long- High ratio of carbon to cow’s digestion of grass branched alkanes and hydrogen more soot Also contains ethane, alkenes when burning propane, isomers of must separated into butane simpler mixtures distributed by (=fractions) –gasoline, underground pipes heating oil energy for heating + fractional distillation – cooking, production of using different boiling electricity points (=condensing pts) – found along w/ deposits of separate mixtures – coal + petroleum petroleum gas, gasoline, kerosene, heating oil, lubricating oil Combustion of fossil fuels complete combustion (burn completely) C3H8 + 5O2 3CO2 + 4H2O (propane + oxygen carbon dioxide + water Burning fossil fuels more carbon dioxide, sulfur dioxide, nitrogen oxides (S, N in fossil fuels + O2)
incomplete combustion (not enough oxygen, so burn incompletely) C3H8 + 7O2 6CO + 8H2O CO (carbon monoxide) = deadly, colorless, odorless inhaled + absorbed by blood stops hemoglobin from taking O2 to cells Tiny particles of C heart + lung problems
acid rain rain is usually acidic (pH 5.6); w/ carbonic acid (H2CO3 from carbon dioxide), sulfuric acid (H2SO4), nitric acid (HNO3) pH of rain lower (=more acidic)