V E R S I O N 2 . 7 1 j
I. IMPROVEMENTS
I.1. Processing of third country lists
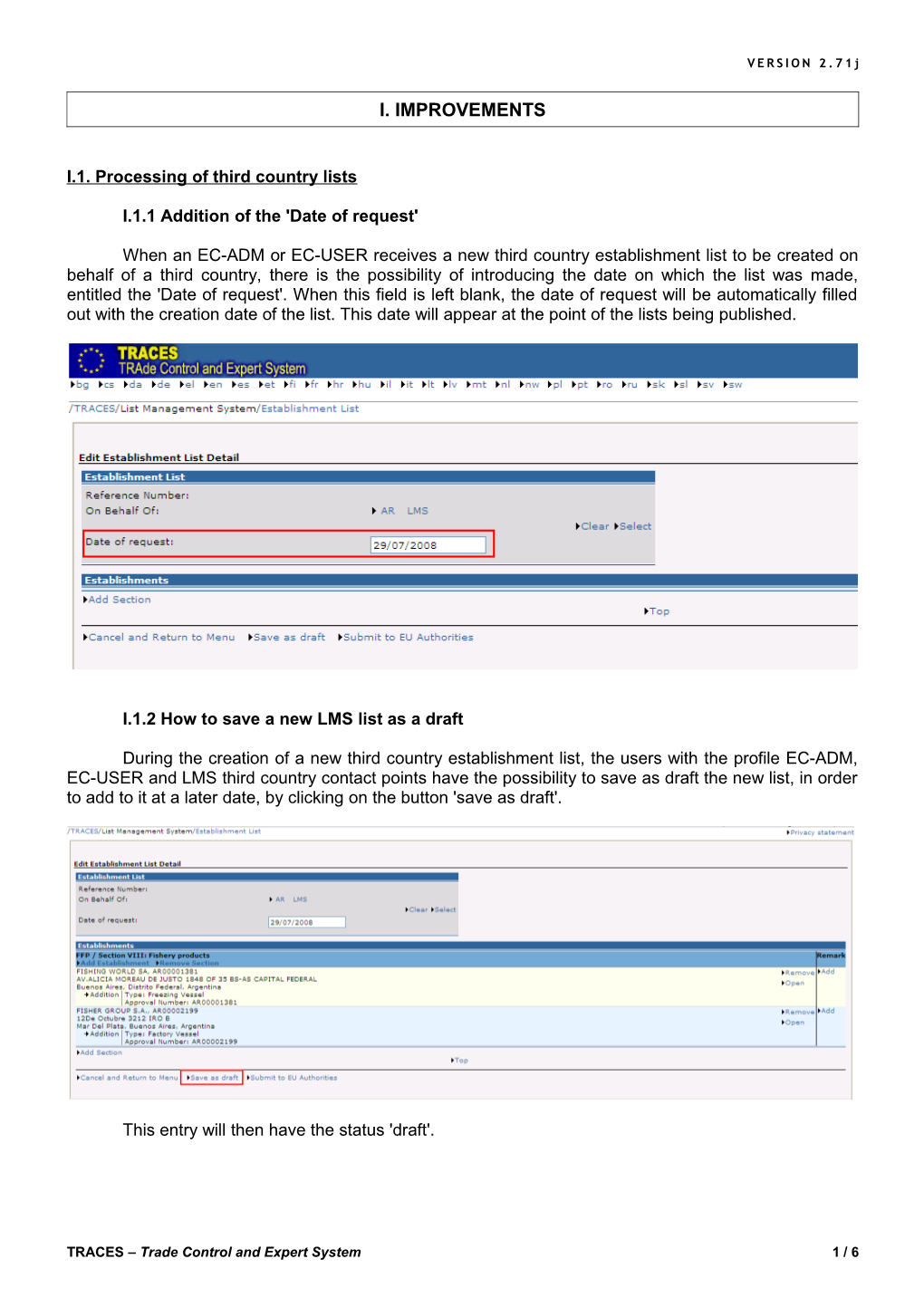
I.1.1 Addition of the 'Date of request'
When an EC-ADM or EC-USER receives a new third country establishment list to be created on behalf of a third country, there is the possibility of introducing the date on which the list was made, entitled the 'Date of request'. When this field is left blank, the date of request will be automatically filled out with the creation date of the list. This date will appear at the point of the lists being published.
I.1.2 How to save a new LMS list as a draft
During the creation of a new third country establishment list, the users with the profile EC-ADM, EC-USER and LMS third country contact points have the possibility to save as draft the new list, in order to add to it at a later date, by clicking on the button 'save as draft'.
This entry will then have the status 'draft'.
TRACES – Trade Control and Expert System 1 / 6 V E R S I O N 2 . 7 1 j
Third countries have the possibility of creating lists with a 'draft' status without taking into account the imposed time to elapse between the submission of two lists to the EC.
I.1.3 Date and status in the search option for new LMS lists
In the menu 'List Management System', the status and the date of the establishment lists which are listed in the search results correspond to the status of the lists on the actual day of checking.
For example: A list which is published and accepted on 28/07/2008 will come into force on 10/08/2008. In the search results this entry will have the status 'Accepted, Published' from 28/07 until 10/08 and will be dated 28/07, the date on which it obtained this status. On 10/08, the status will become 'In force' and the date posted will be 10/08.
TRACES – Trade Control and Expert System 2 / 6 V E R S I O N 2 . 7 1 j
I.1.4 Improvement of data processing when an LMS list is submitted / published
An improvement has been made to reduce the time of data processing of an LMS list.
I.1.5. Authorised third country listings of semen centres and embryo teams
Semen storage centres and semen collection centres are published on the public web site and incorporated into TRACES the same as all other third country establishments which need to comply with the prelisting procedure.
For this purpose, two new sections have been created in TRACES. Section XVII: 'Semen centre' and Section XVIII: 'Embryo team'.
The sections do not separate species, but these are distinguishable in the 'Remarks' column. Authorised third country semen centres for several species are now in a single list.
Four new business types of activity have been added:
Semen collection centre (SCC) and semen storage centre (SSC) within section XVII
Embryo collection team (ECT) and embryo production team (FIV) within section XVIII.
When the specific EC decisions come into force, the semen centres and the embryo teams will be incorporated into the lists. It is thus no longer possible for the codes 051110 (bovine semen) and 05119099 (semen other than that of 051110, embryos, ova) to create organisations in box 10 'establishment' of a CVEDP.
TRACES – Trade Control and Expert System 3 / 6 V E R S I O N 2 . 7 1 j
A new abbreviation has been added for dog semen: 'Can' for 'Canis familiaris'.
Specific categories concerning semen, ova and embryos already exist in TRACES and those distinguishing among the species (for example: bovine, porcine, equine semen centres, etc.) are still active at the moment.
TRACES – Trade Control and Expert System 4 / 6 V E R S I O N 2 . 7 1 j
Eventually, these types of categories will be eliminated but only for third countries.
TRACES – Trade Control and Expert System 5 / 6 V E R S I O N 2 . 7 1 j
II. CERTIFICATION
II.1. IMPORTS
II.1.1. Products of animal origin coming from New Zealand (2006/855/EC)
The IMPORT certificate appearing in annex II of Decision 2003/56/EC has been replaced by that of Decision 2006/855/EC. This certificate also incorporates the modifications relating to the transmissible spongiform encephalopathy required by Regulation 722/2007/EC.
II.1.2. Blood products for the manufacture of technical products (523/2008)
"Blood products, excluding equidae serum for technical purposes" certificate of Regulation 1774/2002 has been replaced by two certificates in Regulation N° 523/2008/EC of 11 June 2008 amending annexes VIII, X and XI to Regulation n°1774/2002/EC of the European Parliament and of the Council as regards the import of blood products for the manufacture of technical products:
- 'Untreated blood samples, with the exclusion of equine blood samples, for use in the manufacture of technical products' (523/2008);
- Treated blood products, excluding of equidae, for technical products (523/2008).
3002 is the nomenclature code linked to these certificates.
TRACES – Trade Control and Expert System 6 / 6