Principles of Chemistry II, CHEM 1212 Dr. Pennington Exam #1 (Chapters 15 and 16 – 100 points) 3/5/08 Name:______
Total: / 75 Prorated: / 100
Show your work. Messy or sloppy work will not be graded. Remember that not all equations on this exam are shown balanced Equations you may find useful:
+ - -14 - pKa = -log Ka [H ][OH ] = 1x10 pOH = -log [OH ] + -14 pH = -log [H ] pH + pOH =14 Ka.Kb = Kw (1x10 )
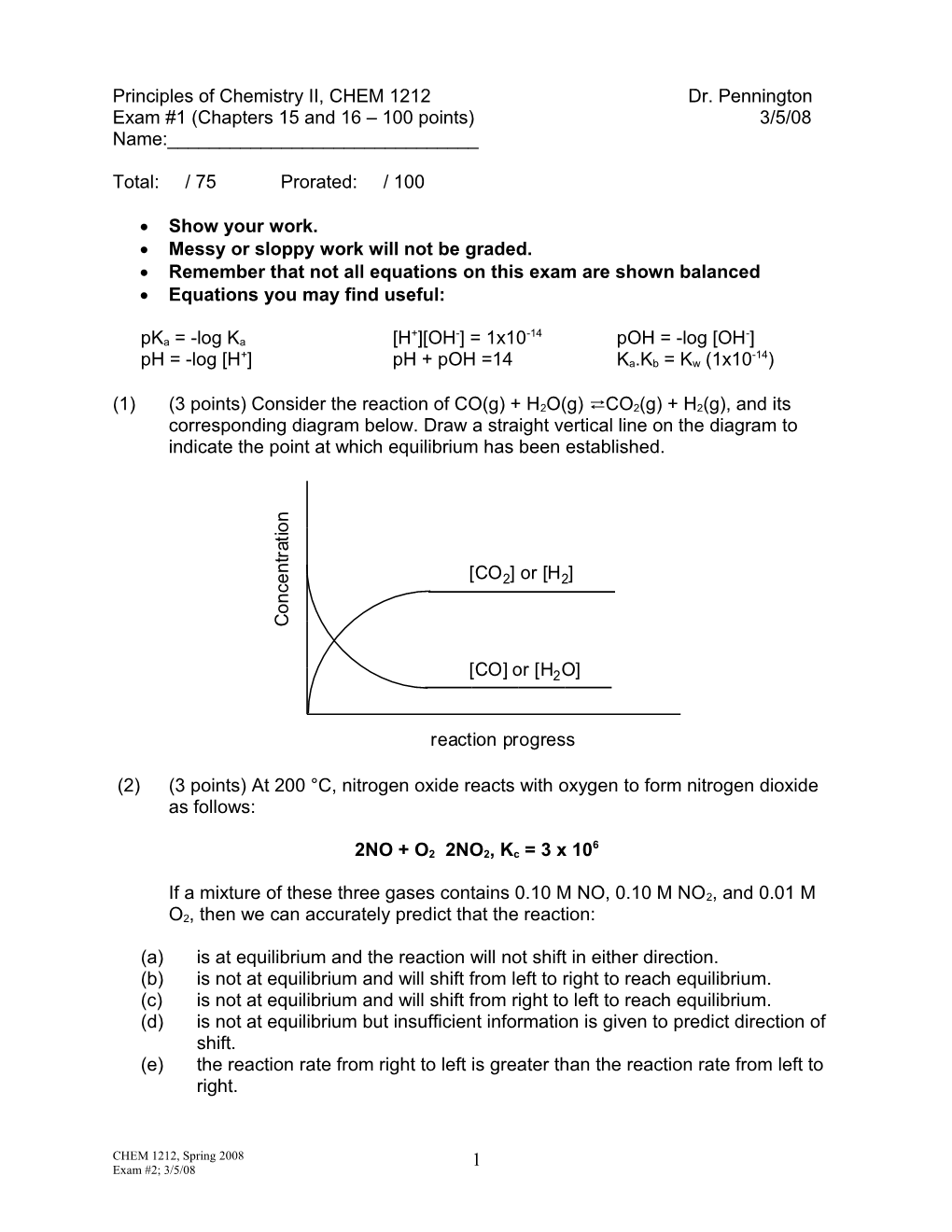
(1) (3 points) Consider the reaction of CO(g) + H2O(g) ⇄ CO2(g) + H2(g), and its corresponding diagram below. Draw a straight vertical line on the diagram to indicate the point at which equilibrium has been established. n o i t a r t
n [CO ] or [H ] e 2 2 c n o C
[CO] or [H2O]
reaction progress
(2) (3 points) At 200 °C, nitrogen oxide reacts with oxygen to form nitrogen dioxide as follows:
6 2NO + O2 2NO2, Kc = 3 x 10
If a mixture of these three gases contains 0.10 M NO, 0.10 M NO2, and 0.01 M O2, then we can accurately predict that the reaction:
(a) is at equilibrium and the reaction will not shift in either direction. (b) is not at equilibrium and will shift from left to right to reach equilibrium. (c) is not at equilibrium and will shift from right to left to reach equilibrium. (d) is not at equilibrium but insufficient information is given to predict direction of shift. (e) the reaction rate from right to left is greater than the reaction rate from left to right.
CHEM 1212, Spring 2008 1 Exam #2; 3/5/08 (3) (3 points) Consider the reaction of CO(g) + H2O(g) ⇄ CO2(g) + H2(g). This reaction has a H = -435 kJ/mol. How will the amount of H2O at equilibrium be affected by increasing the reaction temperature?
(a) [H2O] will increase (b) [H2O] will decrease (c) [H2O] will be unaffected (d) The question cannot be answered, because H2O(g) should not be included in the equilibrium expression.
2- (4) (3 points) "Consider the species A (H2O) and B (HPO4 ). In the reaction where 2- H2O is the acid and HPO4 is the base, which of the following statements is true?"
+ 3- (a) The conjugate base of A is H3O ; the conjugate base of B is PO4 . + - (b) The conjugate acid of A is H3O ; the conjugate base of B is H2PO4 . - - (c) The conjugate base of A is OH ; the conjugate acid of B is H2PO4 . - 3- (d) The conjugate acid of A is OH ; conjugate base of B is PO4 .
(5) (3 points) An aqueous solution of which of the following salts will have the lowest pH?
(a) NaCl (b) NH4NO2 (c) NH4Cl (d) KC2H3O2 (e) LiOH
(6) (3 points) Which of the following is the strongest acid?
-5 (a) Acetic acid (HC2H3O2); Ka = 1.8 x 10 -10 (b) Hydrocyanic acid (HCN); Ka = 6.2 x 10 -5 (c) Benzoic acid (C6H5CO2H); Ka = 6.4 x 10 -4 (d) Formic acid (HCHO2); Ka = 1.8 x 10 -2 (e) Chlorous acid (HClO2); Ka = 1.2 x 10
(7) (4 points) What are the equilibrium expressions for each of the following?
(a) NaHCO3(s) ⇄ Na2CO3(s) + CO2(g) + H2O(g)
(b) Hg(l) + Cl2(g) ⇄ Hg2Cl2(s)
CHEM 1212, Spring 2008 2 Exam #2; 3/5/08 (8) (6 points) Consider the following reaction between nitrogen dioxide and nitric oxide: NO2(g) + NO(g) ⇄ N2O(g) + O2(g)
Originally, the reaction vessel contained the following initial concentrations:
[N2O] = 0.184 M; [O2] = 0.377 M; [NO2] = 0.0560 M; [NO] = 0.294 M.
The concentration of NO2, the only colored gas in this mixture, was monitored by following the intensity of the color. At equilibrium, [NO2] had become 0.118 M. What is the value of Kc for this reaction at this temperature?
(9) (7 points) The equilibrium constant, Kc, for the reaction
SO3(g) + NO(g) ⇄ NO2(g) + SO2(g)
-4 is found to be 5.00 x 10 at a certain temperature. If 0.240 mol of SO3 and 0.240 mol of NO are placed in a 5 L container and allowed to react, what will be the equilibrium concentration of each gas?
CHEM 1212, Spring 2008 3 Exam #2; 3/5/08 (10) (7 points) Equilibrium constants can be used in terms of concentrations or partial pressures. In these cases, the equilibrium constants are shown by Kc and Kp,
respectively. In most cases, Kc Kp. However, in some cases, these two are equal. Answer the following:
(a) What is the formula that relates Kc and Kp together?
(b) Under what conditions is Kc = Kp?
(c) Indicate, for each of the following four reactions, whether Kc = Kp or not.
(i) O3(g) + NO(g) O2(g) + NO2(g) Kc = Kp Yes / No
(ii) H2(g) + NO(g) N2(g) + H2O(g) Kc = Kp Yes / No
(iii) N2(g) + H2(g) NH3(g) Kc = Kp Yes / No
(iv) CH4(g) + 2O2(g) CO2(g) + 2H2O(g) Kc = Kp Yes / No
(11) (8 points) Calculate the following:
(a) pOH of 1.5 M HNO3
+ (b) [H ] of a 0.35 M Cu(OH)2 solution
(c) pH of 0.1 M LiOH
(d) [OH-] of 0.005 M HCl
CHEM 1212, Spring 2008 4 Exam #2; 3/5/08 -4 (12) (8 points) Formic acid (HCHO2) has a Ka of 1.8x10 . What is the pH of a 0.25 M formic acid solution?
2- (13) (6 points) The hydrogenphosphate ion, HPO3 , is an example of a species that is ‘amphoteric’.
(a) What does the term ‘amphoteric’ mean?
2- (b) Provide two equilibrium reactions that illustrate how HPO3 is amphoteric. Hint: include the H+ ion somewhere in each of your two reactions.
Reaction 1:
Reaction 2:
(14) (8 points) Consider the following equilibrium:
2PBr3(g) + 3Cl2(g) ⇄ 2PCl3(g) + 3Br2(g)
What would happen to the position of the equilibrium (if anything) if each of the following changes were made?
(a) Increasing [Br2]
(b) Decreasing [PBr3]
(c) Decreasing the volume of the reaction flask
(d) Adding an alkene to the reaction flask (alkenes react with Br2) CHEM 1212, Spring 2008 5 Exam #2; 3/5/08 (15) (3 points) For the reaction shown below, which box represents where the equililbrium has first been established? Circle your choice.
X + Y ⇄ XY
(a) (b) (c) (d) (e)
CHEM 1212, Spring 2008 6 Exam #2; 3/5/08