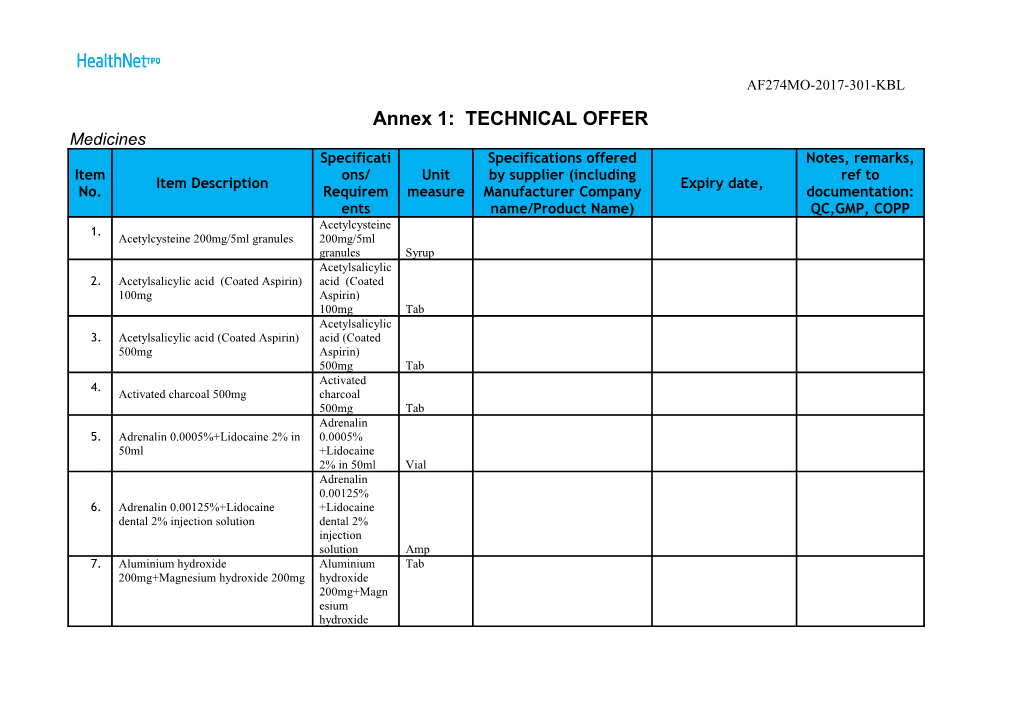
AF274MO-2017-301-KBL Annex 1: TECHNICAL OFFER Medicines Specificati Specifications offered Notes, remarks, Item ons/ Unit by supplier (including ref to Item Description Expiry date, No. Requirem measure Manufacturer Company documentation: ents name/Product Name) QC,GMP, COPP Acetylcysteine 1. Acetylcysteine 200mg/5ml granules 200mg/5ml granules Syrup Acetylsalicylic 2. Acetylsalicylic acid (Coated Aspirin) acid (Coated 100mg Aspirin) 100mg Tab Acetylsalicylic 3. Acetylsalicylic acid (Coated Aspirin) acid (Coated 500mg Aspirin) 500mg Tab Activated 4. Activated charcoal 500mg charcoal 500mg Tab Adrenalin 5. Adrenalin 0.0005%+Lidocaine 2% in 0.0005% 50ml +Lidocaine 2% in 50ml Vial Adrenalin 0.00125% 6. Adrenalin 0.00125%+Lidocaine +Lidocaine dental 2% injection solution dental 2% injection solution Amp 7. Aluminium hydroxide Aluminium Tab 200mg+Magnesium hydroxide 200mg hydroxide 200mg+Magn esium hydroxide AF274MO-2017-301-KBL 200mg Aluminium 8. Aluminium hydroxide 500mg hydroxide 500mg Tab 9. Amiodarone Amiodarone 150mg 150mg Amp 10 Amikacin . Amikacin 100mg/ml, in 2ml 100mg/ml, in 2ml Amp 11 Aminophyllin . Aminophylline 100mg e 100mg Tab 12 Aminophyllin . Aminophylline 25mg/ml, in 10ml e 25mg/ml, in 10ml Amp 13 Amitriptylline . Amitriptylline 25mg 25mg Tab 14 Amoxicillin . Amoxicillin 250mg Cap 250mg Cap Cap 15 Amoxicillin . Amoxicillin 500mg Cap 500mg Cap Cap Amoxicillin 125mg + 16 Amoxicillin 125mg + Clavulanic acid Clavulanic . 31.25mg/5ml, in 60ml bottle, acid 31.25mg/5ml, in 60ml bottle, Susp Amoxicillin 17 500mg+Clavul Amoxicillin 500mg+Clavulanic acid . anic acid 125mg (Co-amoxiclav) 125mg (Co- amoxiclav) Tab AF274MO-2017-301-KBL 18 . Ampicillin 1g Ampicillin 1g Vial Anti-D 19 immunoglobul Anti-D immunoglobulin (human) . in (human) 250mcg per dose, in 1 dose 250mcg per dose, in 1 dose Vial 20 Artemether . Artemether 80mg/ml, 1ml, 80mg/ml, 1ml, Amp 21 Atenolol . Atenolol 100mg 100mg Tab 22 . Atenolol 50mg Atenolol 50mg Tab Atropine 23 sulfate . Atropine sulfate 1mg/ml, in 1ml 1mg/ml, in 1ml Amp Beclomethaso 24 n dipropionate Beclomethason dipropionate 50mcg . 50mcg per per dose, nasal spray dose, nasal spray Spray Benzoic Acid 25 Benzoic Acid 6%+Salicylic acid 3% 6%+Salicylic . topical Oint acid 3% topical Oint Oint 26 Biperiden . Biperiden 5mg/ml 1ml Amp 5mg/ml 1ml Amp Amp 27 Bisacodyl 5mg . Bisacodyl 5mg Tab Tab Tab 28 Bupivacaine 5mg/ml, 4ml Amp Bupivacaine Amp . 5mg/ml, 4ml AF274MO-2017-301-KBL Amp Calcium 29 Gluconate . Calcium Gluconate 10% 10ml Amp 10% 10ml Amp Amp 30 Calamine (Zn0 . Calamine (Zn0 + Fe203) Lotion: 8% + Fe203) Lotion: 8% Lotion 31 Captopril . Captopril 25mg Tab 25mg Tab Tab 32 Carbamazepin . Carbamazepine 200mg Tab e 200mg Tab Tab 33 Cefazolin 1g . Cefazolin 1g Vial Vial Vial 34 Cefotaxime 1g . Cefotaxime 1g Vial + Water Vial + Water Vial 35 Cefotaxime . Cefotaxime 500mg Vial+ Water 500mg Vial+ Water Vial 36 Ceftriaxone 1g . Ceftriaxone 1g Vial + Water Vial + Water Vial 37 Ceftriaxone . Ceftriaxone 500mg Vial +Water 500mg Vial +Water Vial Chlorampheni 38 col 0.5% in Chloramphenicol 0.5% in 2.5ml . 2.5ml bottle, bottle, ophthalmic drop ophthalmic drop Drop 39 Chloramphenicol 0.5% Chlorampheni Drop . +Dexamethasone 0.1% in 10ml bottle, col 0.5% AF274MO-2017-301-KBL +Dexamethaso ne 0.1% in ophthalmic drop 10ml bottle, ophthalmic drop Chlorhexidine 40 Chlorhexidine Digluconate 5% Digluconate . Topical Sol 5% Topical Sol Sol 41 Chloroquine . Chloroquine 150mg Tab 150mg Tab Tab 42 Chloroquine . Chloroquine 50mg/5ml 60ml Syr 50mg/5ml 60ml Syr Syrup Chlorphenami 43 Chlorphenamine maleate 10mg/ml , in ne maleate . 1ml Amp 10mg/ml , in 1ml Amp Amp 44 Chlorphenami . Chlorphenamine maleate 4mg Tab ne maleate 4mg Tab Tab 45 Chlorpromazi . Chlorpromazine 100mg Tab ne 100mg Tab Tab 46 Ciprofloxacin . Ciprofloxacin 2mg/ml 100ml Vial 2mg/ml 100ml Vial Vial 47 Cloxacillin . Cloxacillin 500mg Vial 500mg Vial Vial 48 Dexamethason . Dexamethasone 4mg /ml 1ml Amp e 4mg /ml 1ml Amp Amp 49 Diazepam . Diazepam 5mg Tab 5mg Tab Tab AF274MO-2017-301-KBL Diazepam 50 5mg/ml 2ml Diazepam 5mg/ml 2ml Amp . Amp (Valum/Martin Dow) (Valum/Marti n Dow) Amp 51 Diclofenac . Diclofenac 25mg/ml 3ml Amp 25mg/ml 3ml Amp Amp Diphenhydram 52 Diphenhydramine chloride 14mg/5ml, ine chloride . 90ml Syr 14mg/5ml, 90ml Syr Syrup Dobutamine 53 Dobutamine Injection: 12.5 mg/ml in Injection: 12.5 . 20 ml mg/ml in 20 ml Vial 54 Dopamine . Dopamine 40mg/ml 5ml Amp 40mg/ml 5ml Amp Amp 55 Doxycycline . Doxycycline 100mg Cap 100mg Cap Cap 56 Ephedrine Hcl Ephedrine Hcl 3mg/ml, in 10ml , . 3mg/ml, in Amp 10ml , Amp Amp Epinephrine 57 Epinephrine (Adrenaline) 0.1% in 1ml (Adrenaline) . Amp 0.1% in 1ml Amp Amp 58 Ferrous . Ferrous Sulphate 200mg Tab Sulphate 200mg Tab Tab Ferrous 59 Sulphate Ferrous Sulphate 60mg+Folic Acid . 60mg+Folic 0.4mg Tab Acid 0.4mg Tab Tab AF274MO-2017-301-KBL 60 Flumazenil . Flumazenil 0.1mg/ml, in 10ml , Amp 0.1mg/ml, in 10ml , Amp Amp 61 Fluoxetine . Fluoxetine 20mg Cap 20mg Cap Cap 62 Enoxaparine . Enoxaparine 100mg/ml (LMWH) 100mg/ml (LMWH) Amp 63 Folic Acid . Folic Acid 5mg Tab 5mg Tab Tab Plasma 64 Plasma (Hemaccel), in 500ml bottle, (Hemaccel), in . infusion Sol 500ml bottle, infusion Sol Infusion Sol Furosemide 65 Furosemide 10mg/ml, in 2ml Amp 10mg/ml, in . (Lasix) 2ml Amp (Lasix) Amp Gentamicin 66 0.3% in 5ml Gentamicin 0.3% in 5ml bottle, . bottle, ophthalmic drop ophthalmic drop Drop 67 Gentamicin . Gentamicin 20mg/ml, 2ml Amp 20mg/ml, 2ml Amp Amp 68 Gentamicin . Gentamicin 40mg/ml, 2ml Amp 40mg/ml, 2ml Amp Amp Glucose 10% 69 Glucose 10% 1000ml IV infusion+IV 1000ml IV . Set infusion+IV Set Infusion Sol 70 Glucose 10% 500ml IV infusion+IV Glucose 10% Infusion Sol . Set 500ml IV infusion+IV AF274MO-2017-301-KBL Set Glucose 4% +Sodium 71 Chloride Glucose 4%+Sodium Chloride 0.18% . 0.18% 500ml 500ml IV infusion+IV Set IV infusion+IV Set Infusion Sol Glucose 5% 72 Glucose 5% 1000ml IV infusion+IV 1000ml IV . Set infusion+IV Set Infusion Sol Glucose 5% 73 Glucose 5% 500ml IV infusion+IV 500ml IV . Set infusion+IV Set Infusion Sol 74 Glucose 25% . Glucose 25% 20ml 20ml Vial Glucose 5% +Sodium 75 Glucose 5%+Sodium Chloride 0.9% Chloride 0.9% . 1000ml IV infusion+IV Set 1000ml IV infusion+IV Set Infusion Sol Glucose 5% +Sodium 76 Glucose 5%+Sodium Chloride 0.9% Chloride 0.9% . 500ml IV infusion+IV Set 500ml IV infusion+IV Set Infusion Sol 77 Glyceryl . Glyceryl trinitrate 10 mg/10 ml trinitrate 10 mg/10 ml Amp 78 Griseofulvin . Griseofulvin 500mg Tab 500mg Tab Tab AF274MO-2017-301-KBL 79 Haloperidol . Haloperidol 5mg Tab 5mg Tab Tab 80 Haloperidol . Haloperidol 5mg/ml, 1ml Amp 5mg/ml, 1ml Amp Amp 81 Hydrochloroth . Hydrochlorothiazide 50mg Tab iazide 50mg Tab Tab 82 Hydrocortison . Hydrocortisone Acetate 1% 15g Oint e Acetate 1% 15g Oint Oint Hydrogen 83 peroxide 6% Hydrogen peroxide 6% topical . topical Solution 500ml Solution 500ml Solution 84 Ibuprofen . Ibuprofen 200mg Tab 200mg Tab Tab 85 Ibuprofen 400 . Ibuprofen 400 mg Tab mg Tab Tab Insulin 86 (regular) . 100IU/ml, Insulin (regular) 100IU/ml, 10ml Vial 10ml Vial Vial Insulin 87 Humuline(70/ . Insulin Humuline(70/30) 100IU/ml, 30) 100IU/ml, 10ml Vial 10ml Vial Vial 88 Isoflurane in . 250 milliliter, Isoflurane in 250 milliliter, gas gas bottl 89 Ketamin . Ketamin 50mg/ml 10ml Vial 50mg/ml 10ml Vial Vial AF274MO-2017-301-KBL 90 Ketoconazole Ketoconazole , 20% 30g, topical . , 20% 30g, cream topical cream Oint 91 . Labetolol Labetolol Amp 92 Lidocaine 2% in . Lidocaine 2% in 15g tube, topical gel 15g tube, topical gel Tube 93 Lidocaine 2% in Lidocaine 2% in 50ml Vial . 50ml Vial Vial 94 Lindane . Lindane Lotion: 1% Lotion: 1% bottl Magnesium 95 Sulfate Magnesium Sulfate 500mg/ml (50%) . 500mg/ml 10ml Amp (50%) 10ml Amp Amp Mannitol 20% 96 500ml . Mannitol 20% 500ml Infusion +IV Infusion +IV Set Set Infusion Sol 97 Methyldopa . Methyldopa 250mg Tab 250mg Tab Tab 98 Methylergometr Methylergometrine 0.2mg Tab . ine 0.2mg Tab Tab Methylrosanili 99 ne (Gentian Methylrosaniline (Gentian Violet) 1% . Violet) 1% in in 25ml bottle, topical Sol 25ml bottle, topical Sol Sol 10 Metocloprami 0. Metoclopramide 5mg/ml, in 2ml Amp de 5mg/ml, in 2ml Amp Amp AF274MO-2017-301-KBL 10 Metronidazole 1. Metronidazole 200mg Tab 200mg Tab Tab 10 Metronidazole 2. Metronidazole 400mg Tab 400mg Tab Tab Metronidazole 10 Metronidazole 5mg/ml, in 100ml 5mg/ml, in 3. bottle, infusion Sol 100ml bottle, infusion Sol Sol 10 Misoprostol 4. Misoprostol 200mcg Tab 200mcg Tab Tab Morphine 10 (sulfate,hydroc Morphine (sulfate,hydrochloride) 5. hloride) 10mg/ml, in 1ml Amp 10mg/ml, in 1ml Amp Amp 10 6. Multivitamin Multivitamin Tab Tab Tab Naloxone 10 Naloxone 400mcg/ml (0.4mg/ml) in 400mcg/ml 7. 1ml Amp (0.4mg/ml) in 1ml Amp Amp Neostigmine 10 Neostigmine methylsulfate2.5mg/ml, methylsulfate2 8. in 10ml Amp .5mg/ml, in 10ml Amp Amp 10 Nitrofurantoin 9. Nitrofurantoin 100mg Tab/Cap 100mg Tab/Cap Tab Nystatin 11 Nystatin 100,000IU 30ml Oral 100,000IU 0. Topical Drop 30ml Oral Topical Drop Drop 11 Nystatin 100,000IU Oral Tab Nystatin Tab 1. 100,000IU AF274MO-2017-301-KBL Oral Tab Nystatin 11 100,000IU Nystatin 100,000IU Vaginal 2. Vaginal Tab/ovule suppository Tab/ovule suppository Tab 11 Nystatin 3. Nystatin 500,000IU Tab, oral 500,000IU Tab, oral Tab 11 Omeprazole 4. Omeprazole 40mg/ml 40mg/ml Vial 11 Oxytocine 5. Oxytocine 10IU/ml in 1ml Amp 10IU/ml in 1ml Amp Amp Pancuronium 11 Bromide Pancuronium Bromide (Pavulon) 6. (Pavulon) 2mg/ml, 2ml Amp 2mg/ml, 2ml Amp Amp 11 Paracetamol 7. Paracetamol 100mg Tab 100mg Tab Tab 11 Paracetamol 8. Paracetamol 120mg/5ml, 60ml Syr 120mg/5ml, 60ml Syr Syrup Penicillin 11 Penicillin Benzyl (Peni G crystal) Benzyl (Peni 9. 5MU Vial G crystal) 5MU Vial Vial 12 Penicillin V 0. Penicillin V Potassium 250mg Tab Potassium 250mg Tab Tab 12 Penicillin V 1. Penicillin V Potassium 500mg Potassium 500mg Tab AF274MO-2017-301-KBL 12 Pethidine 2. 100mg/ml, Pethidine 100mg/ml, 1ml Amp 1ml Amp Amp 12 Phenobarbital 3. Phenobarbital 100mg Tab 100mg Tab Tab 12 Phenobarbital 4. Phenobarbital 15mg Tab 15mg Tab Tab 12 Phenobarbital 5. 200mg/ml, Phenobarbital 200mg/ml, 1ml Amp 1ml Amp Amp Phytomenadio 12 ne (Vit K) Phytomenadione (Vit K) 10mg/ml, 6. 10mg/ml, 5ml 5ml Amp (50mg/5ml) Amp (50mg/5ml) Amp Polymyxin 10000IU+Baci 12 Polymyxin 10000IU+Bacitracin tracin 500IU 7. 500IU per gram, in 6g tube, per gram, in ophthalmic oint 6g tube, ophthalmic oint Tube Povidone- 12 Iodine 10% 8. Povidone-Iodine 10% Topical Sol Topical Sol 450ml 450ml Sol 12 Prednisolone 9. Prednisolone 0.5% 5ml eye Drops 0.5% 5ml eye Drops Drop 13 0. Prednisolone Prednisolone 5mg 5mg Tab 13 1. Primaquine Primaquine 15mg Tab 15mg Tab Tab AF274MO-2017-301-KBL 13 2. Promethazine Promethazine 25mg Tab 25mg Tab Tab 13 3. Propoful Propoful Vial 13 Propranolol 20mg Propranolol 4. 20mg Tab 13 5. Propranolol Propranolol 40mg 40mg Tab 13 6. Promethazine Promethazine 25 mg/ml 25 mg/ml Amp Quinine 13 Quinine dihydrochloride 300mg/ml, dihydrochlorid 7. in 2ml e 300mg/ml, in 2ml Amp 13 Ranitidine 8. Ranitidine 150mg 150mg Tab 13 Ranitidine 9. Ranitidine 25mg/ml, in 2ml 25mg/ml, in 2ml Amp 14 Retinol (Vit 0. Retinol (Vit A) 100,000 IU A) 100,000 IU Tab 14 Retinol (Vit 1. Retinol (Vit A) 200,000 IU A) 200,000 IU Cap Ringer lactate 14 1000ml IV 2. Ringer lactate 1000ml IV infusion+IV infusion+IV Set Set Infusion Sol 14 Ringer lactate 500ml IV infusion+IV Ringer lactate Infusion Sol 3. Set 500ml IV infusion+IV AF274MO-2017-301-KBL Set 14 Salbutamol 4. 2mg/5ml, in Salbutamol 2mg/5ml, in 60ml 60ml Syrup 14 Salbutamol 5. Salbutamol 4mg 4mg Tab Salbutamol 14 5mg/ml in Salbutamol 5mg/ml in 30ml 6. 30ml Inhalation Sol (for Nublizer) Inhalation Sol (for Nublizer) Sol 14 Silver 7. Silver Sulfadiazine 1% Oint 250g Sulfadiazine 1% Oint 250g Can 14 Sodium 8. Sodium Bicarbonate 8.4% 10ml Bicarbonate 8.4% 10ml Amp Sodium Chloride 0.9% 14 1000ml IV Sodium Chloride 0.9% 1000ml IV 9. infusion infusion (Normal Saline) + IV Set (Normal Saline) + IV Set Infusion Sol Sodium Chloride 0.9% 15 Sodium Chloride 0.9% 500ml IV 500ml IV 0. infusion (Normal Saline)+IV Set infusion (Normal Saline)+IV Set Infusion Sol 15 Streptokinase 1. Streptokinase 1.5 MU 1.5 MU Vial 15 Sulphacetamid 2. Sulphacetamide 10% 10ml Eye e 10% 10ml Eye Drop AF274MO-2017-301-KBL 15 Sulphacetamid 3. Sulphacetamide 20% 10ml Eye e 20% 10ml Eye Drop 15 Suxamethoniu 4. Suxamethonium 50mg/ml 2ml m 50mg/ml 2ml Amp 15 Tetracaine 5. Tetracaine 0.5% 15ml Eye 0.5% 15ml Eye Drop 15 Tetracycline 6. Tetracycline Eye 1% Oint Eye 1% Oint Oint 15 Thiopental 7. Thiopental Sodium 500mg Sodium 500mg Vial 15 Tramadol 8. Tramadol 100mg 100mg Tab 15 Tramadol 9. Tramadol 50mg/ml, in 2ml 50mg/ml, in 2ml Amp 16 Tranexamic 0. Tranexamic acid 50mg/ml, in 5ml acid 50mg/ml, in 5ml Amp 16 Trihexyphenid 1. Trihexyphenidyl 2mg yl 2mg Tab Valproic Acid 16 Valproic Acid (Sodium valproate) (Sodium 2. 200mg valproate) 200mg Tab Valproic Acid 16 Valproic Acid (Sodium valproate) (Sodium 3. 500mg valproate) 500mg Tab 16 Vecuronium Bromide 10mg/ml, in Vecuronium Vial 4. 10ml Bromide 10mg/ml, in AF274MO-2017-301-KBL 10ml Vecuronium 16 Bromide 5. Vecuronium Bromide 4mg/ml, in 2ml 4mg/ml, in 2ml Amp 16 Vencomycine 6. Vencomycine 1g 1g Vial 16 Verapamil 7. Verapamil 2.5mg 2.5mg Amp 16 Water for Injection 10ml Water for 8. Injection 10ml Amp
Evaluation Committee
Notes:______AF274MO-2017-301-KBL
Annex 2: PRICE BREAKDOWN Page No 01 of______PUBLICATION REFERENCE: AF274MO-2017- 301-KBL
PRICE BREAKDOWN A B D E F G Unit Cost Currency With Delivery Total Cost with According to ITT Item (Incoterm and delivery (Incoterm Quantity Item Description NO. Destinations and Destinations Specified in Specified in ITT) ITT) 1. Acetylcysteine 200mg/5ml 8 granules 2. Acetylsalicylic acid (Coated 8,000 Aspirin) 100mg 3. Acetylsalicylic acid (Coated 16,000 Aspirin) 500mg 4. Activated charcoal 500mg 20,000 5. Adrenalin 0.0005%+Lidocaine 2% 1,000 in 50ml 6. Adrenalin 0.00125%+Lidocaine 800 dental 2% injection solution 7. 260,000 Aluminium hydroxide 200mg+Magnesium hydroxide AF274MO-2017-301-KBL 200mg 8. Aluminium hydroxide 500mg 120,000 9. Amiodarone 150mg 200 10. Amikacin 100mg/ml, in 2ml 8,000 11. Aminophylline 100mg 5,000 12. Aminophylline 25mg/ml, in 10ml 150 13. Amitriptylline 25mg 180,000 14. Amoxicillin 250mg Cap 100,000 15. Amoxicillin 500mg Cap 200,000 16. Amoxicillin 125mg + Clavulanic 200 acid 31.25mg/5ml, in 60ml bottle, 17. Amoxicillin 500mg+Clavulanic 12,000 acid 125mg (Co-amoxiclav) 18. Ampicillin 1g 16,000 19. Anti-D immunoglobulin (human) 32 250mcg per dose, in 1 dose 20. Artemether 80mg/ml, 1ml, 2,300 21. Atenolol 100mg 6,600 22. Atenolol 50mg 8,000 23. Atropine sulfate 1mg/ml, in 1ml 8,000 24. Beclomethason dipropionate 20 50mcg per dose, nasal spray 25. Benzoic Acid 6%+Salicylic acid 800 3% topical Oint AF274MO-2017-301-KBL 26. Biperiden 5mg/ml 1ml Amp 160 27. Bisacodyl 5mg Tab 1,000 28. Bupivacaine 5mg/ml, 4ml Amp 1,300 29. Calcium Gluconate 10% 10ml 5,000 Amp 30. Calamine (Zn0 + Fe203) Lotion: 260 8% 31. Captopril 25mg Tab 4,000 32. Carbamazepine 200mg Tab 54,000 33. Cefazolin 1g Vial 4,200 34. Cefotaxime 1g Vial + Water 8,500 35. Cefotaxime 500mg Vial+ Water 4,000 36. Ceftriaxone 1g Vial + Water 48,000 37. Ceftriaxone 500mg Vial +Water 22,000 38. Chloramphenicol 0.5% in 2.5ml 900 bottle, ophthalmic drop Chloramphenicol 0.5% 39. +Dexamethasone 0.1% in 10ml 900 bottle, ophthalmic drop 40. Chlorhexidine Digluconate 5% 100 Topical Sol 41. Chloroquine 150mg Tab 8,000 42. Chloroquine 50mg/5ml 60ml Syr 200 43. Chlorphenamine maleate 10mg/ml 1,000 , in 1ml Amp AF274MO-2017-301-KBL 44. Chlorphenamine maleate 4mg Tab 48,000 45. Chlorpromazine 100mg Tab 12,000 46. Ciprofloxacin 2mg/ml 100ml Vial 16,000 47. Cloxacillin 500mg Vial 12,000 48. Dexamethasone 4mg /ml 1ml Amp 4,000 49. Diazepam 5mg Tab 12,000 50. Diazepam 5mg/ml 2ml Amp 5,000 (Valum/Martin Dow) 51. Diclofenac 25mg/ml 3ml Amp 32,000 52. Diphenhydramine chloride 500 14mg/5ml, 90ml Syr 53. Dobutamine Injection: 12.5 800 mg/ml in 20 ml 54. Dopamine 40mg/ml 5ml Amp 1,400 55. Doxycycline 100mg Cap 2,000 56. Ephedrine Hcl 3mg/ml, in 10ml , 500 Amp 57. Epinephrine (Adrenaline) 0.1% in 3,600 1ml Amp 58. Ferrous Sulphate 200mg Tab 280,000 59. Ferrous Sulphate 60mg+Folic 420,000 Acid 0.4mg Tab 60. Flumazenil 0.1mg/ml, in 10ml , 100 Amp 61. Fluoxetine 20mg Cap 15,000 62. 400 Enoxaparine 100mg/ml (LMWH) AF274MO-2017-301-KBL
63. Folic Acid 5mg Tab 40,000 64. Plasma (Hemaccel), in 500ml 1,600 bottle, infusion Sol 65. Furosemide 10mg/ml, in 2ml Amp 18,000 (Lasix) 66. Gentamicin 0.3% in 5ml bottle, 800 ophthalmic drop 67. Gentamicin 20mg/ml, 2ml Amp 4,000 68. Gentamicin 40mg/ml, 2ml Amp 1,000 69. Glucose 10% 1000ml IV 3,200 infusion+IV Set 70. Glucose 10% 500ml IV 3,200 infusion+IV Set 71. Glucose 4%+Sodium Chloride 9,000 0.18% 500ml IV infusion+IV Set 72. Glucose 5% 1000ml IV 12,000 infusion+IV Set 73. Glucose 5% 500ml IV 7,200 infusion+IV Set 74. Glucose 25% 20ml 1,600 75. Glucose 5%+Sodium Chloride 3,600 0.9% 1000ml IV infusion+IV Set 76. Glucose 5%+Sodium Chloride 2,400 0.9% 500ml IV infusion+IV Set 77. Glyceryl trinitrate 10 mg/10 ml 90 78. Griseofulvin 500mg Tab 3,000 79. Haloperidol 5mg Tab 10,000 80. Haloperidol 5mg/ml, 1ml Amp 100 AF274MO-2017-301-KBL 81. Hydrochlorothiazide 50mg Tab 100 82. Hydrocortisone Acetate 1% 15g 200 Oint 83. Hydrogen peroxide 6% topical 200 Solution 500ml 84. Ibuprofen 200mg Tab 80,000 85. Ibuprofen 400 mg Tab 135,000 86. Insulin (regular) 100IU/ml, 10ml 80 Vial 87. Insulin Humuline(70/30) 80 100IU/ml, 10ml Vial 88. 80 Isoflurane in 250 milliliter, gas 89. Ketamin 50mg/ml 10ml Vial 6,500 90. Ketoconazole , 20% 30g, topical 700 cream 91. 250 Labetolol 92. Lidocaine 2% in 15g tube, topical gel 2,600 93. Lidocaine 2% in 50ml Vial 100 94. Lindane Lotion: 1% 550 95. Magnesium Sulfate 500mg/ml 1,000 (50%) 10ml Amp 96. Mannitol 20% 500ml Infusion +IV 1,200 Set 97. Methyldopa 250mg Tab 3,000 98. Methylergometrine 0.2mg Tab 2,000 99. 1,200 Methylrosaniline (Gentian Violet) AF274MO-2017-301-KBL 1% in 25ml bottle, topical Sol 100 Metoclopramide 5mg/ml, in 2ml . Amp 12,000 101 . Metronidazole 200mg Tab 40,000 102 . Metronidazole 400mg Tab 40,000 103 Metronidazole 5mg/ml, in 100ml . bottle, infusion Sol 32,000 104 . Misoprostol 200mcg Tab 12,000 105 Morphine (sulfate,hydrochloride) . 10mg/ml, in 1ml Amp 300 106 . 100,000 Multivitamin Tab 107 Naloxone 400mcg/ml (0.4mg/ml) . in 1ml Amp 2,000 108 Neostigmine . methylsulfate2.5mg/ml, in 10ml 7,000 Amp 109 . Nitrofurantoin 100mg Tab/Cap 4,000 110 Nystatin 100,000IU 30ml Oral . Topical Drop 600 111 . Nystatin 100,000IU Oral Tab 200 AF274MO-2017-301-KBL 112 Nystatin 100,000IU Vaginal . Tab/ovule suppository 400 113 . Nystatin 500,000IU Tab, oral 1,200 114 . Omeprazole 40mg/ml 1,600 115 . Oxytocine 10IU/ml in 1ml Amp 80,000 116 Pancuronium Bromide (Pavulon) . 2mg/ml, 2ml Amp 2,000 117 . Paracetamol 100mg Tab 48,000 118 . Paracetamol 120mg/5ml, 60ml Syr 3,000 119 Penicillin Benzyl (Peni G crystal) . 5MU Vial 7,200 120 . Penicillin V Potassium 250mg Tab 16,000 121 . Penicillin V Potassium 500mg 16,000 122 . 20 Pethidine 100mg/ml, 1ml Amp 123 . Phenobarbital 100mg Tab 8,000 124 6,000 Phenobarbital 15mg Tab . AF274MO-2017-301-KBL
125 . Phenobarbital 200mg/ml, 1ml 4,000 Amp 126 Phytomenadione (Vit K) 10mg/ml, . 5ml Amp (50mg/5ml) 1,200 127 Polymyxin 10000IU+Bacitracin . 500IU per gram, in 6g tube, 500 ophthalmic oint 128 . Povidone-Iodine 10% Topical Sol 5,600 450ml 129 . Prednisolone 0.5% 5ml eye Drops 80 130 . 1,600 Prednisolone 5mg 131 . 1,000 Primaquine 15mg Tab 132 . 7,000 Promethazine 25mg Tab 133 . 600 Propoful 134 Propranolol 20mg . 4,000 135 . 3,000 Propranolol 40mg 136 . 50 Promethazine 25 mg/ml AF274MO-2017-301-KBL 137 Quinine dihydrochloride . 300mg/ml, in 2ml 600 138 . Ranitidine 150mg 16,000 139 . Ranitidine 25mg/ml, in 2ml 7,200 140 . Retinol (Vit A) 100,000 IU 4,000 141 . Retinol (Vit A) 200,000 IU 1,000 142 . Ringer lactate 1000ml IV 42,000 infusion+IV Set 143 Ringer lactate 500ml IV . infusion+IV Set 20,000 144 . 1,600 Salbutamol 2mg/5ml, in 60ml 145 . Salbutamol 4mg 4,000 146 Salbutamol 5mg/ml in 30ml . Inhalation Sol (for Nublizer) 500 147 . Silver Sulfadiazine 1% Oint 250g 2,000 148 . Sodium Bicarbonate 8.4% 10ml 50 149 32,000 Sodium Chloride 0.9% 1000ml IV . infusion (Normal Saline) + IV Set AF274MO-2017-301-KBL
150 Sodium Chloride 0.9% 500ml IV . infusion (Normal Saline)+IV Set 17,000 151 . Streptokinase 1.5 MU 20 152 . Sulphacetamide 10% 10ml Eye 800 153 . Sulphacetamide 20% 10ml Eye 800 154 . Suxamethonium 50mg/ml 2ml 5,000 155 . Tetracaine 0.5% 15ml Eye 160 156 . Tetracycline Eye 1% Oint 1,600 157 . Thiopental Sodium 500mg 100 158 . Tramadol 100mg 100 159 . Tramadol 50mg/ml, in 2ml 2,400 160 . Tranexamic acid 50mg/ml, in 5ml 2,500 161 . Trihexyphenidyl 2mg 8,000 AF274MO-2017-301-KBL 162 Valproic Acid (Sodium valproate) . 200mg 1,000 163 Valproic Acid (Sodium valproate) . 500mg 1,000 164 Vecuronium Bromide 10mg/ml, in . 10ml 600 165 Vecuronium Bromide 4mg/ml, in . 2ml 4,000 166 . Vencomycine 1g 300 167 . Verapamil 2.5mg 90 168 Water for Injection 10ml . 90,000 Grand Total AFN AF274MO-2017-301-KBL
ANNEX 3: COMPANY INFORMATION TENDER PARTICIPANT.
Publication reference: AF274MO-2017-301KBL
A: HealthNet TPO Afghanistan office, House-144, Street-5, Sello Road District-3, Dehnaw, Dehburi, Kabul, Afghanistan
One signed original form must be supplied, together with the number of copies specified in the Instruction to Tenderers. The form must include a signed declaration using the annexed format from each legal entity making the application. Any additional documentation (brochure, letter, etc) sent with the form will not be taken into consideration.Applications being submitted by aconsortium (i.e. either a permanent, legally-established grouping or a grouping which has been constituted informally for a specific tender procedure) must follow the instructions applicable to the consortium leader and its members. An economic operator may, where appropriate and for a particular contract, rely on the capacities of other entities, regardless of the legal nature of the links which it has with them. It must in that case prove to the contracting authority that it will have at its disposal the resources necessary for performance of the contract, for example by producing an undertaking on the part of those entities to place those resources at its disposal. Such entities, for instance the parent company of the economic operator, must respect the same rules of eligibility and notably that of nationality, as the economic operator. 1 SUBMITTED BY
Name(s) of tenderer(s) Nationality2 Leader
Member
Etc …
1 add/delete additional lines for members as appropriate. Note that a subcontractor is not considered to be a member for the purposes of this tender procedure. Subsequently, the data of the subcontractor must not appear in the data related to the economic, financial and professional capacity. If this tender is being submitted by an individual tenderer, the name of the tenderer should be entered as 'leader' (and all other lines should be deleted) 2Country in which the legal entity is registered AF274MO-2017-301-KBL 2 CONTACT PERSON
Name
Address
Telephone
Fax E-mail
3 ECONOMIC AND FINANCIAL CAPACITY
Please complete the following table of financial data3 based on your annual accounts and your latest projections. If annual accounts are not yet available for this year or last year, please provide your latest estimates, clearly identifying estimated figures in italics. Figures in all columns must be on the same basis to allow a direct, year-on-year comparison to be made (or, if the basis has changed, an explanation of the change must be provided as a footnote to the table). Any clarification or explanation which is judged necessary may also be provided.
Financial data Year Last year This year Next year Average 4 before last USD USD USD USD USD Annual turnover 5, excluding this contract Cash and cash equivalents6 at beginning of year Net cash from / (used in) operating, investing & financing activities7 excluding future contracts Net forecast cash from/ (used in) future contracts, excluding this contract Cash and cash equivalents6 at end of year [i.e., the sum of the above three rows]
3 if this application is being submitted by a consortium, the data in the table above must be the sum of the data in the corresponding tables in the declarations provided by the consortium members – see point 7 of this tender form for a supply contract.
4 Amounts entered in the 'Average' column must be the mathematical average of the amounts entered in the four preceding columns of the same row.
5 The gross inflow of economic benefits (cash, receivables, other assets) arising from the ordinary operating activities of the enterprise (such as sales of goods, sales of services, interest, royalties, and dividends) during the year.
6 Cash and cash equivalents comprise cash on hand and demand deposits, together with short-term, highly liquid investments that are readily convertible to a known amount of cash and that are subject to an insignificant risk of changes in value. An investment normally meets the definition of a cash equivalent when it has a maturity of three months or less from the date of acquisition. Equity investments are normally excluded, unless they are in substance a cash equivalent (e.g. preferred shares acquired within three months of their specified redemption date). Bank overdrafts which are repayable on demand and AF274MO-2017-301-KBL which form an integral part of an enterprise's cash management are also included as a component of cash and cash equivalents.
7 Operating activities are the main revenue-producing activities of the enterprise that are not investing or financing activities, so operating cash flows include cash received from customers and cash paid to suppliers and employees. Investing activities are the acquisition and disposal of long-term assets and other investments that are not considered to be cash equivalents. Financing activities are activities that alter the equity capital and borrowing structure of the enterprise. Interest and dividends received and paid may be classified as operating, investing, or financing cash flows, provided that they are classified consistently from period to period. Cash flows arising from taxes on income are normally classified as operating, unless they can be specifically identified with financing or investing activities. AF274MO-2017-301-KBL 4 EXPERIENCE Please complete a table using the format below to summarize the major relevant medicines contracts carried out in the course of the past 3 years by the legal entity or entities making this tender. The number of references to be provided must not exceed 5 for the entire tender Ref # Project Title … (maximum 5) Name of legal Country Overall Proportio No of staff Name of Origin of Dates Name of entity supply n provided client funding members if value ( USD) supplied any by legal entity (%)
Detailed Related services provided description of supply … …
5 TENDERER'S DECLARATION(S)
To be completed and signed by the tenderer (including one from each member in a consortium). In response to your letter of invitation to tender for the above contract, we, the undersigned, hereby declare that: 1 We have examined and accept in full the content of the dossier for invitation to tender No <……………………………….> of
Name and first name: <[…………………………………………………………………>
Duly authorized to sign this tender on behalf of: <…………………………………………………………………………………… …>
Place and date: <…………………………………………………………….………….>]
Stamp of the firm/company:
This tender includes the following annexes:
[Numbered list of annexes with titles] AF274MO-2017-301-KBL Annex 4: Declaration of eligibility.
………………………… (name) representing ……………………………………… (name of organization declares by signing this document that the points hereunder mentioned are not applicable to the organization or will be in the near future:
That the organization is bankrupt or being wound up, are having their affairs administered by the courts, have entered into an arrangement with creditors, have suspended business activities or are in any analogous situation arising from a similar procedure provided for in national legislation or regulations; That the organization is the subject of proceedings for a declaration of bankruptcy, for winding-up, for administration by the courts, for an arrangement with creditors or for any similar procedure provided for in national legislation or regulations; That the organization has been convicted of an offense concerning professional conducted by a judgment which has the force of re judicatory; That the organization is guilty of grave professional misconduct proven by any means which the Contracting Authority can justify; That the organization does not have unfulfilled obligations relating to the payment of taxes/other applicable dues i.e. social security contributions in accordance with the legal provisions of the country where they are established; That the organization has no unfulfilled obligations relating to the payment of taxes in accordance with the legal provisions of the country where they are established That the organization is not guilty of serious misrepresentation in supplying the information required by the Contracting Authority as a condition of participation in an invitation to tender or contract; That the organization has not been declared to be in serious breach of contract for failure to comply with obligations in connection with another contract with the same Contracting Authority or another contract financed with SEHAT funds; That the organization is not in one of the situations allowing exclusion referred to the Ethics Clauses in connection with the Tender of the contract.
As so declared …………………………………………………..(city, date)
Signature......
Name: ………………………………......
Position:...... AF274MO-2017-301-KBL
Annex 5: Tender Guarantee Form
Tender Guarantee (Bank Guarantee)
[The Bank shall fill in this Bank Guarantee Form in accordance with the instructions indicated.] ______[Bank’s Name, and Address of Issuing Branch or Office] Beneficiary: ______[Name and Address of Contracting authority] Date: ______TENDER GUARANTEE No.: ______We have been informed that [name of the Tenderer] (hereinafter called "the Tenderer") has submitted to you its tender dated (hereinafter called "the Tender") for the execution of [Supply of medicines] under Invitation for Tenderer No.AF274MO-2017-301-KBL. Furthermore, we understand that, according to your conditions, tenders must be supported by a tender guarantee. At the request of the Tenderer, we [name of Bank] hereby irrevocably undertake to pay you any sum or sums not exceeding in total an amount of [amount in figures] ([amount in words]) upon receipt by us of your first demand in writing accompanied by a written statement stating that the Tenderer is in breach of its obligation(s) under the tender conditions, because the Tenderer: (a) has withdrawn its Tender during the period of bid validity specified by the Tenderer in the Form of Tender; or (b) having been notified of the acceptance of its Tender by the Contracting Authority during the period of tender validity, (i) fails or refuses to execute the Contract Form; or (ii) fails or refuses to furnish the performance guarantee, if required, in accordance with the Instructions to Tenderers.
This guarantee will expire: (a) if the Tenderer is the successful tenderer, upon our receipt of copies of the contract signed by the Tenderer and the performance guarantee issued to you upon the instruction of the Tenderer; or (b) if the Tenderer is not the successful tenderer, upon the earlier of (i) our receipt of a copy of your notification to the Tenderer of the name of the successful Tenderer; or (ii) twenty- eight days after the expiration of the Tenderer’s Tender.
Consequently, any demand for payment under this guarantee must be received by us at the office on or before that date.
This guarantee is subject to the Uniform Rules for Demand Guarantees, ICC Publication No. 458. ______[signature(s)]