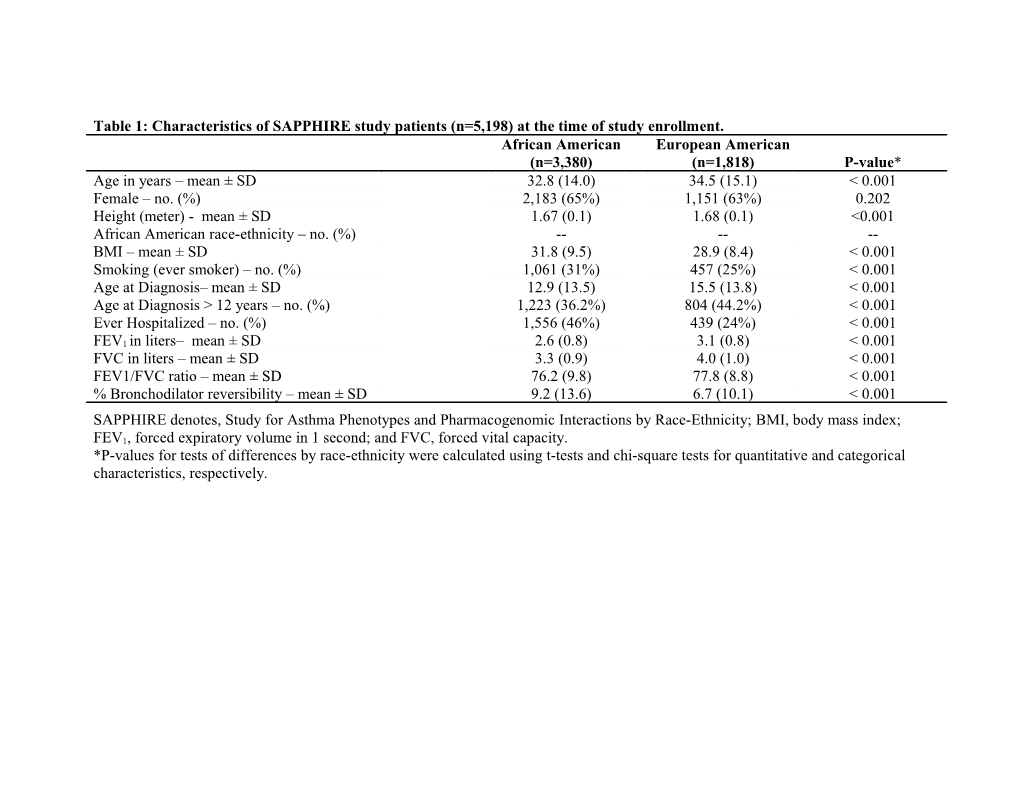
Table 1: Characteristics of SAPPHIRE study patients (n=5,198) at the time of study enrollment. African American European American (n=3,380) (n=1,818) P-value* Age in years – mean ± SD 32.8 (14.0) 34.5 (15.1) < 0.001 Female – no. (%) 2,183 (65%) 1,151 (63%) 0.202 Height (meter) - mean ± SD 1.67 (0.1) 1.68 (0.1) <0.001 African American race-ethnicity – no. (%) ------BMI – mean ± SD 31.8 (9.5) 28.9 (8.4) < 0.001 Smoking (ever smoker) – no. (%) 1,061 (31%) 457 (25%) < 0.001 Age at Diagnosis– mean ± SD 12.9 (13.5) 15.5 (13.8) < 0.001 Age at Diagnosis > 12 years – no. (%) 1,223 (36.2%) 804 (44.2%) < 0.001 Ever Hospitalized – no. (%) 1,556 (46%) 439 (24%) < 0.001 FEV1 in liters– mean ± SD 2.6 (0.8) 3.1 (0.8) < 0.001 FVC in liters – mean ± SD 3.3 (0.9) 4.0 (1.0) < 0.001 FEV1/FVC ratio – mean ± SD 76.2 (9.8) 77.8 (8.8) < 0.001 % Bronchodilator reversibility – mean ± SD 9.2 (13.6) 6.7 (10.1) < 0.001 SAPPHIRE denotes, Study for Asthma Phenotypes and Pharmacogenomic Interactions by Race-Ethnicity; BMI, body mass index; FEV1, forced expiratory volume in 1 second; and FVC, forced vital capacity. *P-values for tests of differences by race-ethnicity were calculated using t-tests and chi-square tests for quantitative and categorical characteristics, respectively. Table 2: Assessment of factors for association with nocturnal asthma and potential differences by race-ethnicity*. African European All subjects American American OR† 95% CI OR† 95% CI OR† 95%CI Pint‡ Age at 1.02 1.02- 1.03 1.03- 1.02 1.01- 1.000 Enrollm 1.03 1.04 1.03 ent Race/Et 2.95 2.61------hnicity 3.34 (Africa n Americ an) Sex 1.61 1.43- 1.64 1.43- 1.91 1.30- 0.940 (Female 1.82 1.89 2.00 ) Height 0.22 0.13- 0.32 0.16- 0.23 0.08- 0.620 0.40 0.64 0.68 BMI 1.04 1.03- 1.03 1.02- 1.05 1.04- <0.001 1.05 1.03 1.07 Smokin 2.51 2.22- 2.71 2.32- 2.00 1.60- 0.028 g (ever 2.83 3.16 2.51 smoker) Age at 1.01 1.00- 1.01 1.01- 1.01 1.00- 0.230 Diagnos 1.01 1.02 1.02 is Age at 1.28 1.15- 1.49 1.29- 1.31 1.06- 0.378 Diagnos 1.44 1.71 1.61 is > 12 years Ever 2.22 1.98- 1.78 1.55- 2.17 1.73- 0.142 Hospita 2.49 2.04 2.73 lized FEV1 0.47 0.43- 0.52 0.48- 0.54 0.47- 0.668 0.50 0.57 0.62 FVC 0.60 0.57- 0.68 0.63- 0.67 0.60- 0.917 0.64 0.73 0.75 FEV1/F 0.96 0.95- 0.96 0.95- 0.97 0.96- 0.164 VC 0.96 0.97 0.98 ratio % 1.03 1.03- 1.03 1.02- 1.05 1.04- 0.002 Bronch 1.04 1.03 1.06 odilator reversib ility Note: P-values for all of the associations were < 0.001, with the exception of age at diagnosis (p=0.020) and age at diagnosis > 12 years (p=0.011) for the European American stratum. BMI, body mass index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; OR, odds ratio; 95%CI, 95% confidence interval; Pint, p-value from a 1 degree of freedom likelihood ratio test of the multiplicative interaction between self-reported race/ethnicity and each variable. *The outcome variable for the association analyses was nocturnal asthma defined as having any night with disrupted sleep due to asthma in the four weeks preceding the enrollment date for each participant. †All odds ratios represent the uni-variable relationship with nocturnal asthma. ‡The p-value for the likelihood ratio test of interaction between race-ethnicity and each variable in a bi-variable model which includes a covariate for self-reported race-ethnicity. Table E1: Multivariable association models of nocturnal asthma including all factors and following stepwise variable selection*. All Covariates Forward Selection† Covariate OR 95% CI P OR 95%CI P Age at Enrollment 1.01 1.00–1.01 0.003 1.01 1.00–1.01 0.005 Race/Ethnicity (African American) 2.48 2.12-2.89 <0.001 2.56 2.24-2.93 <0.001 Sex (Female) 1.29 1.10-1.53 0.003 1.27 1.08-1.49 0.005 Height 1.60 0.62-4.13 0.335 1.83 0.74-4.56 0.192 BMI 1.02 1.01–1.03 <0.001 1.02 1.01–1.03 <0.001 Smoking (ever smoker) 1.98 1.73–2.27 <0.001 2.00 1.74–2.29 <0.001 Age at Diagnosis > 12 years 1.08 0.93–1.25 0.331 --‡ -- -- Ever Hospitalized 1.63 1.44–1.86 <0.001 1.62 1.43–1.84 <0.001 FEV1 0.34 0.17–0.66 0.002 0.72 0.63-0.81 <0.001 FVC 1.83 1.10–3.03 0.020 ------FEV1/FVC ratio 1.02 1.00–1.05 0.072 ------% Bronchodilator reversibility 1.02 1.01–1.03 <0.001 1.02 1.01–1.03 <0.001
BMI, body mass index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; OR, odds ratio; 95%CI, 95% confidence interval; P, p-value from a 1 degree of freedom likelihood ratio test each variable. *The outcome variable for the association analyses was nocturnal asthma defined as having any night with disrupted sleep due to asthma in the four weeks preceding the enrollment date for each participant. †Forward stepwise used for variable selection. ‡Not retained in the final model.
Table 3: Factors associated with nocturnal asthma following stepwise selection among race-ethnic groups* African American† European American† Covariate OR 95% CI P OR 95%CI P Age at Enrollment 1.01 1.01-1.02 <0.001 1.00 1.00-1.01 0.620 Sex (Female) 1.32 1.09-1.61 0.006 1.32 0.97-1.79 0.075 Height 1.30 0.42-4.03 0.644 1.52 0.27-8.46 0.633 BMI 1.01 1.01-1.02 <0.001 1.04 1.03-1.05 <0.001 Smoking (ever smoker) 2.13 1.80-2.52 <0.001 1.55 1.22-1.97 <0.001 Age at Diagnosis --‡ ------Age at Diagnosis > 12 years ------Ever Hospitalized 1.56 1.35-1.81 <0.001 1.75 1.37-2.23 <0.001 FEV1 0.55 0.41-0.74 <0.001 0.75 0.62-0.93 0.009 FVC 1.29 1.09-1.65 0.043 ------FEV1/FVC ratio ------% Bronchodilator reversibility 1.01 1.01-1.02 <0.001 1.03 1.02-1.05 <0.001
BMI, body mass index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; OR, odds ratio; 95%CI, 95% confidence interval; P, p-value from a 1 degree of freedom likelihood ratio test each variable. *The outcome variable for the association analyses was nocturnal asthma defined as having any night with disrupted sleep due to asthma in the four weeks preceding the enrollment date for each participant. †Forward stepwise used for variable selection for the race-ethnicity specific models. ‡Not retained in the final model. Table 4: Causal mediation analysis results evaluating FEV1 and FVC as potential mediating trait between West African ancestry and nocturnal asthma* among African American SAPPHIRE subjects†.
Average Causal Mediation Effect % Mediated Mediato % West African r Ancestry Quintiles Estimate 95%CI Estimate 95%CI P % P
th th -5 -3 -4 FEV1 >20 -40 0.002 6.1*10 - 5.1*10 0.021 -4.5*10 - 0.04 0.06 9.0 0.08 >40th-60th 0.004 5.4*10-5 - 0.01 0.042 2.1*10-3 - 0.04 0.04 8.9 0.07 >60th-80th 0.007 -1.1*10-4 - 0.016 0.063 4.0*10-3 - 0.12 0.03 8.8 0.07 >80th 0.009 -1.9*10-4 - 0.2 0.081 5.3*10-3 - 0.16 0.04 8.7 0.08
FVC 20th-40th 0.003 5.0*10-4 - 0.01 0.020 -1.2*10-4 - 0.04 0.05 12.4 0.04 40th-60th 0.006 9.8*10-4 - 0.01 0.040 -2.5*10-3 - 0.08 0.06 12.7 0.05 60th-80th 0.009 1.5*10-3 - 0.02 0.060 3.4*10-4 - 0.12 0.05 12.7 0.03 >80th 0.012 2.1*10-3 - 0.006 0.079 -9.8*10-4 - 0.16 0.05 13.0 0.04
Notes: All mediation effects were estimated using the subjects in the lowest quintile of percent West African ancestry (≤ 73.7%) as the “control” group and each successive higher quintile as the “treatment” group. The confidence intervals for both the mediation and direct effect estimates were based on the non-parametric bootstrap with 1,500 samples. The mediation effect was estimated using a linear model of lung function (either FEV1 or FVC) and a logistic model for the binary outcome of nocturnal asthma. *Nocturnal asthma was defined as having any night with disrupted sleep due to asthma in the four weeks preceding the enrollment date for each participant.
Figure 1: Percentage of SAPPHIRE participants reporting nocturnal asthma symptoms by race/ethnicity*. *The number of nights with asthma-related sleep disturbance over the month preceding study enrollment. Supplementary Figure 1: Distribution of percent West African ancestry for SAPPHIRE African Americans*.
*1,040 SAPPHIRE African Americans with genome-wide genotyping.