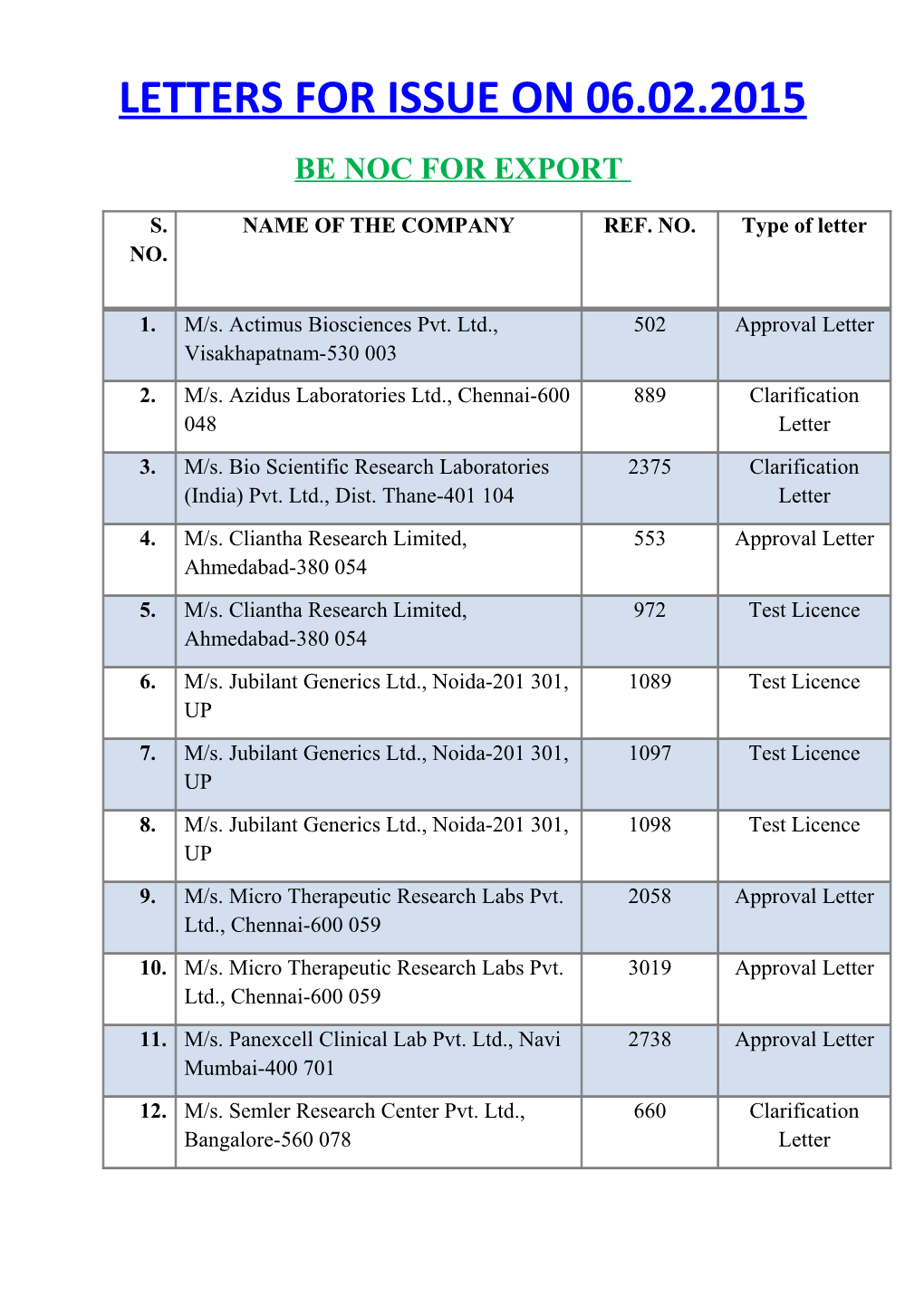
LETTERS FOR ISSUE ON 06.02.2015 BE NOC FOR EXPORT
S. NAME OF THE COMPANY REF. NO. Type of letter NO.
1. M/s. Actimus Biosciences Pvt. Ltd., 502 Approval Letter Visakhapatnam-530 003 2. M/s. Azidus Laboratories Ltd., Chennai-600 889 Clarification 048 Letter 3. M/s. Bio Scientific Research Laboratories 2375 Clarification (India) Pvt. Ltd., Dist. Thane-401 104 Letter 4. M/s. Cliantha Research Limited, 553 Approval Letter Ahmedabad-380 054 5. M/s. Cliantha Research Limited, 972 Test Licence Ahmedabad-380 054 6. M/s. Jubilant Generics Ltd., Noida-201 301, 1089 Test Licence UP 7. M/s. Jubilant Generics Ltd., Noida-201 301, 1097 Test Licence UP 8. M/s. Jubilant Generics Ltd., Noida-201 301, 1098 Test Licence UP 9. M/s. Micro Therapeutic Research Labs Pvt. 2058 Approval Letter Ltd., Chennai-600 059 10. M/s. Micro Therapeutic Research Labs Pvt. 3019 Approval Letter Ltd., Chennai-600 059 11. M/s. Panexcell Clinical Lab Pvt. Ltd., Navi 2738 Approval Letter Mumbai-400 701 12. M/s. Semler Research Center Pvt. Ltd., 660 Clarification Bangalore-560 078 Letter 13. M/s. Shasun Pharmaceuticals Ltd., 51217 Clarification Puducherry-605 014 Letter 14. M/s. Veeda Clinical Research Pvt. Ltd., 269 Clarification Ahmedabad-380 015 Letter 15. M/s. Watson Pharma Pvt. Ltd., Navi 813 Clarification Mumbai-400 614 Letter 16. M/s. Watson Pharma Pvt. Ltd., Navi 826 Clarification Mumbai-400 614 Letter 17. M/s. Watson Pharma Pvt. Ltd., Navi 2602 Clarification Mumbai-400 614 Letter
WRITTEN CONFIRMATION
S. No Firm Name Type of Letter 1. CTX life Sciences Pvt. Ltd, Gujarat WC Certificate 2. Alembic Pharmaceutical Ltd, Gujarat WC Certificate 3. Sarv Biolab Pvt Ltd, Himachal Pradesh WC Certificate 4. Dr, Reddy’s Labs Ltd, Unit VI, A.P. WC Certificate 5. Neuland Labs, Unit –I, Mendak, A.P. WC Certificate 6. Malladi Drugs, Unit V, Reniguntta, A.P. WC Certificate 7. Hospira Healthcare India Ltd, Tamilnadu NOC 8. Intas Pharmaceuticals Ltd, Ahmedabad NOC 9. M/s Eskay Iodine Pvt. Ltd., Jhagadia, Gujarat WC Certificate and Letter
GLOBAL CLINICAL TRIAL
Sr. Name of Firm Reference No. Type of Letter No.
1. M/s. George Clinical 51301 Clarification Letter India 2. M/s. AstraZeneca - Clarification Letter 3. M/s. Maya Clinicals - Clarification Letter 4. M/s. Klinera 767/768 TL Approval letters
REGISRATION
S.NO. NAME OF DIARY NO. TYPE OF APPLICANT LETTER 1. ETHACHEM 42708 APPROVAL LETTER 2. ARCHERCHEM 847 APPROVAL LETTER 3. RANBAXY 44269 CLARIFICATION LABORATORIES LTD. LETTER 4. BAXTER INDIA PVT. 43030 CLARIFICATION LTD. LETTER
IMPORT
S.NO. NAME OF DIARY NO. TYPE OF APPLICANT LETTER 1. MEDOPHARM 2372 AMENDED LETTER 2. NANDLAL 2200 AMENDED BANKATLLAL LETTER PVT.LTD. 3. RELY CHEM 1553 AMENDED LETTER 4. ALEMBIC 44283 AMENDED PHARMACEUTICALS LETTER LTD. 5. ANANTACO 586,1214 02 ENTERPRISES PVT. CLARFICATION LTD. LETTER 6. UCB INDIA PVT. LTD. 1117 7. PFIZER PRODUCTS NIL 02 LETTER INDIA PVT. LTD. 8. YAKSH 1912 CLARIFICATION PHARMA/KYOWA LETTER HAKKO BIO INDIA PVT. LTD.
LETTER PERTAINING TO MISC
S.NO. NAME OF DIARY NO. TYPE OF APPLICANT LETTER 1. THE NORTHERN 48183 LETTER MEDICAL SERVIECES
NEW DRUG
S.NO NAME OF APPLICANT DIARY TYPE OF . NO. LETTER 1. M/s. Virbac Animal Health India Pvt. - Query Letter Ltd
VACCINE DIVISION
S. No. COMPANY NAME Dy. No. STATUS
1 M/s Human Biological Institute Clarification Letter Ltd SEC MEETING LETTER
S. No. COMPANY NAME Dy. No. STATUS
1 M/s Sanofi Pasteur India Pvt, Invitation letter ltd 2 M/s Bharat Biotech Invitation letter International ltd 3 M/s Cadila Pharmaceutical ltd Invitation letter
4 M/s Sinopharma India Pvt, ltd Invitation letter
5 M/s Human Biological Institute Invitation letter
6 M/s Novartis Healthcare Pvt, Invitation letter ltd 7 M/s Synergy Diagnostics Invitation letter
8 M/s Dr. Reddy Laboratories Invitation letter Ltd 9 M/s FDC ltd Invitation letter
10 M/s GSK Pharmaceutical ltd Invitation letter