Ion Master Sheet
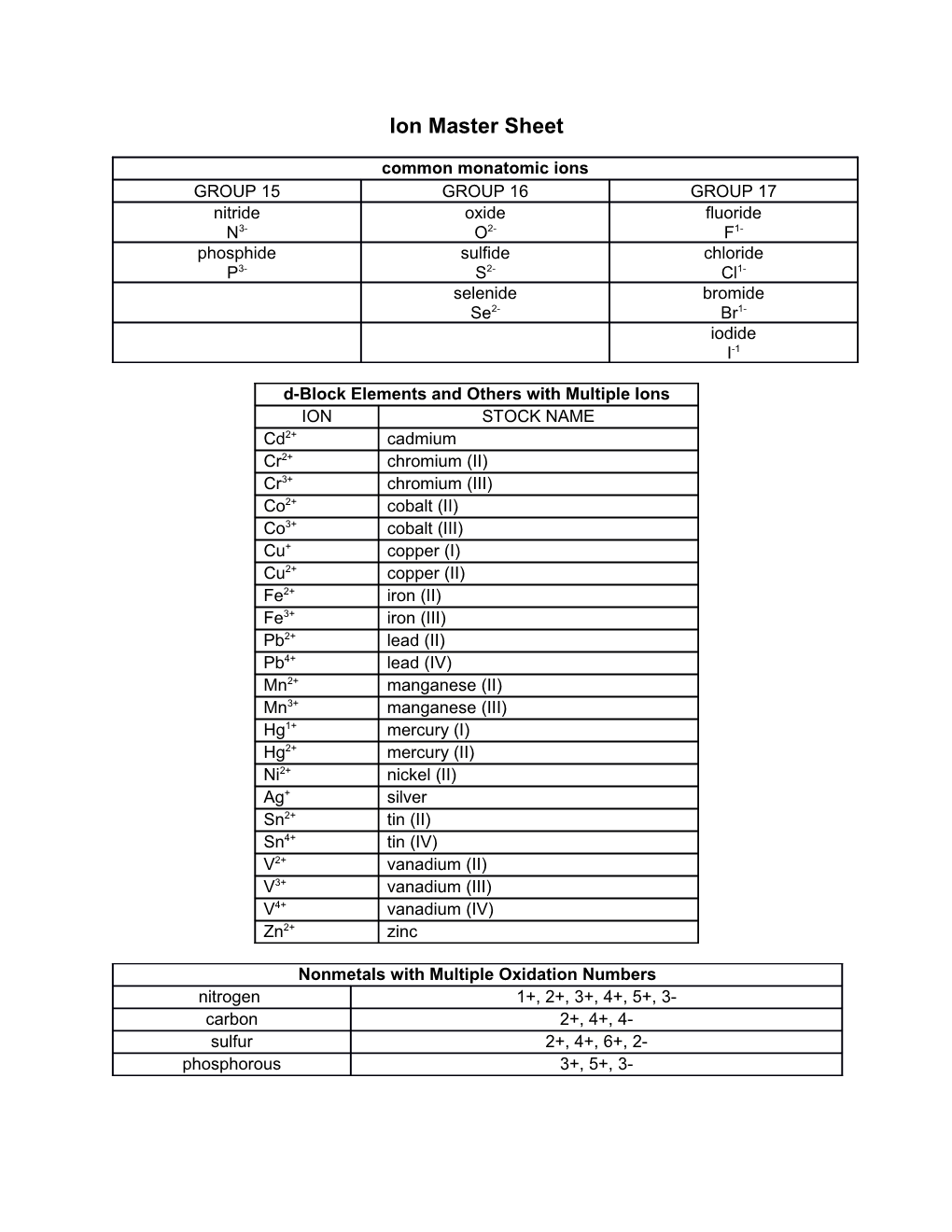
common monatomic ions GROUP 15 GROUP 16 GROUP 17 nitride oxide fluoride N3- O2- F1- phosphide sulfide chloride P3- S2- Cl1- selenide bromide Se2- Br1- iodide I-1
d-Block Elements and Others with Multiple Ions ION STOCK NAME Cd2+ cadmium Cr2+ chromium (II) Cr3+ chromium (III) Co2+ cobalt (II) Co3+ cobalt (III) Cu+ copper (I) Cu2+ copper (II) Fe2+ iron (II) Fe3+ iron (III) Pb2+ lead (II) Pb4+ lead (IV) Mn2+ manganese (II) Mn3+ manganese (III) Hg1+ mercury (I) Hg2+ mercury (II) Ni2+ nickel (II) Ag+ silver Sn2+ tin (II) Sn4+ tin (IV) V2+ vanadium (II) V3+ vanadium (III) V4+ vanadium (IV) Zn2+ zinc
Nonmetals with Multiple Oxidation Numbers nitrogen 1+, 2+, 3+, 4+, 5+, 3- carbon 2+, 4+, 4- sulfur 2+, 4+, 6+, 2- phosphorous 3+, 5+, 3- Common Polyatomic Ions 1+ 1- 2- 3-
ammonium NH4 acetate CH3COO hydrogen phosphate phosphite PO3 HPO4
chlorate ClO3 oxalate C2O4 phosphate PO4
chlorite ClO2 sulfite SO3 arsenate AsO4
cyanide CN sulfate SO4
hydroxide OH carbonate CO3
hypochlorite ClO chromate CrO4
iodate IO3 dichromate Cr2O7
nitrate NO3 silicate SiO3
nitrite NO2 selenate SeO4
perchlorate ClO4 hexafluorosilicate SiF6
permanganate MnO4 tertraborate B4O7
dihydrogen phosphate peroxide O2 H2PO4
hydrogen sulfite tartrate C4H4O6 (bisulfite) HSO3
hydrogen sulfate thiosulfate S2O3 (bisulfate) HSO4 hydrogen carbonate (bicarbonate) HCO3
bromate BrO3
Acids Molecular Compound Prefixes Binary Acids: mono 1 begin with hydro- di 2 end with –ic acid tri 3 tetra 4 Oxyacids: penta 5 Replace –ate with -ic hexa 6 Replace –ite with -ous hepta 7 octa 8 nona 9 deca 10