Electronic supplement Material: icm_2007-00653R2 The risk associated with hyperoncotic colloids in patients with shock Frédérique Schortgen, Emmanuelle Girou, Nicolas Deye, Laurent Brochard
DEVELOPMENT OF PROPENSITY SCORE
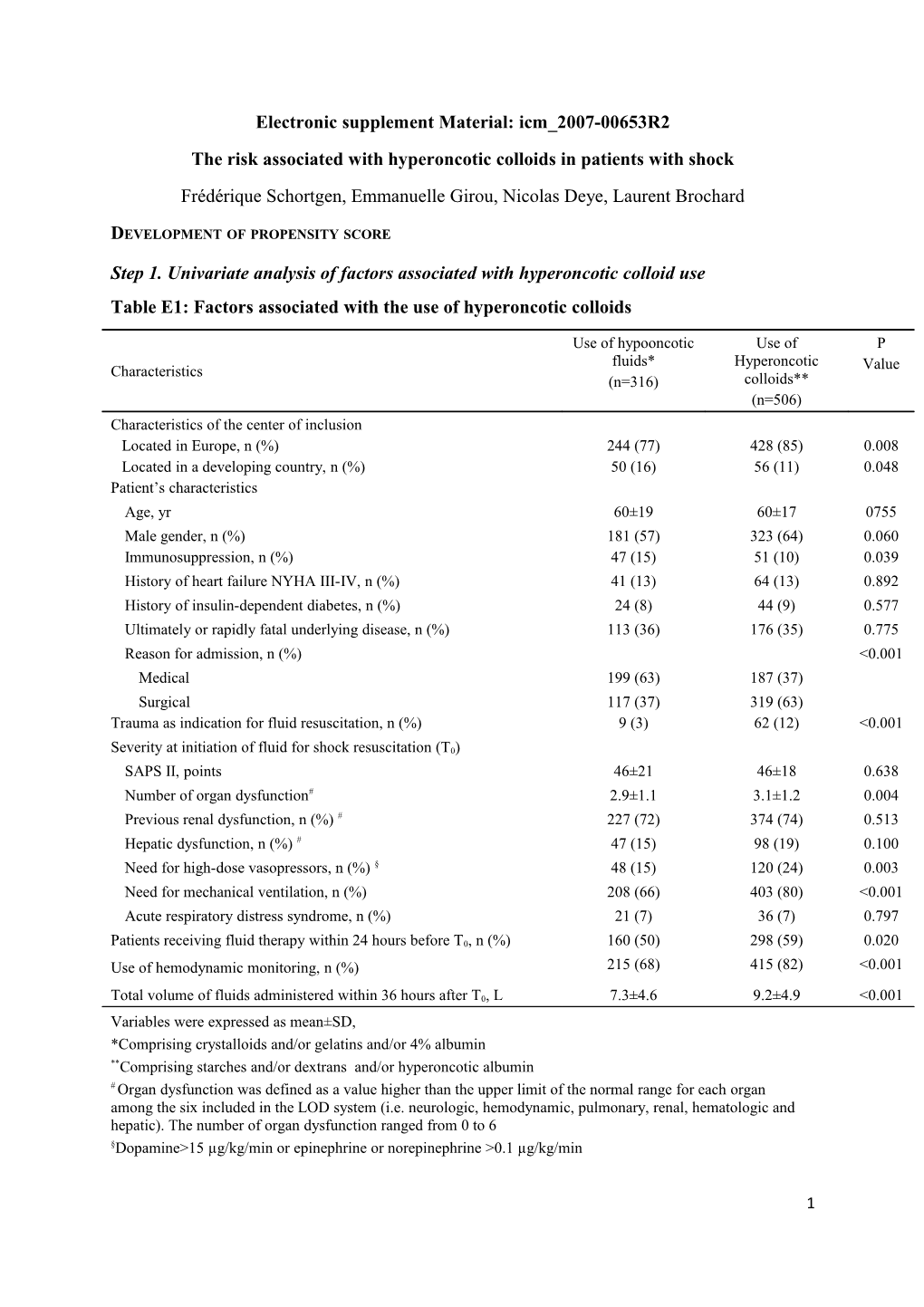
Step 1. Univariate analysis of factors associated with hyperoncotic colloid use Table E1: Factors associated with the use of hyperoncotic colloids
Use of hypooncotic Use of P fluids* Hyperoncotic Characteristics Value (n=316) colloids** (n=506) Characteristics of the center of inclusion Located in Europe, n (%) 244 (77) 428 (85) 0.008 Located in a developing country, n (%) 50 (16) 56 (11) 0.048 Patient’s characteristics Age, yr 60±19 60±17 0755 Male gender, n (%) 181 (57) 323 (64) 0.060 Immunosuppression, n (%) 47 (15) 51 (10) 0.039 History of heart failure NYHA III-IV, n (%) 41 (13) 64 (13) 0.892 History of insulin-dependent diabetes, n (%) 24 (8) 44 (9) 0.577 Ultimately or rapidly fatal underlying disease, n (%) 113 (36) 176 (35) 0.775 Reason for admission, n (%) <0.001 Medical 199 (63) 187 (37) Surgical 117 (37) 319 (63) Trauma as indication for fluid resuscitation, n (%) 9 (3) 62 (12) <0.001
Severity at initiation of fluid for shock resuscitation (T0) SAPS II, points 46±21 46±18 0.638 Number of organ dysfunction# 2.9±1.1 3.1±1.2 0.004 Previous renal dysfunction, n (%) # 227 (72) 374 (74) 0.513 Hepatic dysfunction, n (%) # 47 (15) 98 (19) 0.100 Need for high-dose vasopressors, n (%) § 48 (15) 120 (24) 0.003 Need for mechanical ventilation, n (%) 208 (66) 403 (80) <0.001 Acute respiratory distress syndrome, n (%) 21 (7) 36 (7) 0.797
Patients receiving fluid therapy within 24 hours before T0, n (%) 160 (50) 298 (59) 0.020 Use of hemodynamic monitoring, n (%) 215 (68) 415 (82) <0.001
Total volume of fluids administered within 36 hours after T0, L 7.3±4.6 9.2±4.9 <0.001 Variables were expressed as mean±SD, *Comprising crystalloids and/or gelatins and/or 4% albumin **Comprising starches and/or dextrans and/or hyperoncotic albumin # Organ dysfunction was defined as a value higher than the upper limit of the normal range for each organ among the six included in the LOD system (i.e. neurologic, hemodynamic, pulmonary, renal, hematologic and hepatic). The number of organ dysfunction ranged from 0 to 6 §Dopamine>15 µg/kg/min or epinephrine or norepinephrine >0.1 µg/kg/min
1 Step 2. Calculation of the propensity scores To develop the propensity score, we included in a separate stepwise logistic regression analysis all factors that differed among the hyperoncotic colloids and other groups (either crystalloids only or hypooncotic colloids), using a more liberal significance criterion of P<.15 (Table E1). With hyperoncotic colloid use as the dependent variable, we fitted a model predicting the “propensity” of hyperoncotic colloid use. The following equations were used to derive the propensity score for hyperoncotic colloids use: (a) X= -1.2230 + (center of inclusion located in Europe x 0.5086) + (Trauma x 1.4824) - (medical admission x 1.0018) + (number of organ dysfunction x 0.1921) + (Total volume of fluid x 0.0875) (b) Propensity Score = eX/(1+eX)
The mean propensity score was 0.53 with a range of 0.15 to 0.99. Patients’ cohort was divided into quintiles based on the propensity to receive hyperoncotic colloids (see Table 2). The probability that a patient would receive hyperoncotic colloids (as opposed to crystalloids only or hypooncotic colloids) increased from quintile 1 to quintile 5. Patients within a quintile were similar in their likelihood to receive hyperoncotic colloids. As a validation of the propensity score, the balance of all covariates within each quintile was examined. The prevalence of all covariates was consistent between hyperoncotic colloid and other fluids groups within each quintile. Table E2. Propensity scores for hyperoncotic colloid use by quintiles Hyperoncotic colloid Crystalloid only / hypooncotic colloid Quintiles No. of patients Median (IQR) score No. of patients Median (IQR) score 1 56 0.32 (0.28-0.34) 108 0.29 (0.26-0.32) 2 88 0.42 (0.39-0.44) 77 0.41 (0.38-0.43) 3 95 0.53 (0.51-0.56) 69 0.53 (0.50-0.55) 4 127 0.63 (0.61-0.66) 38 0.62 (0.60-0.64) 5 140 0.77 (0.82-0.86) 24 0.74 (0.69-0.82) IQR indicates interquartile range.
2 ANALYSIS ON THE IMPACT OF THE VOLUME OF ARTIFICIAL HYPERONCOTIC COLLOIDS ON RENAL EVENT OCCURRENCE
In the subgroups of patients resuscitated using artificial hyperoncotic fluids (dextran and/or starches, n=401) we compared the incidence of adverse renal event according to the volume of artificial hyperoncotic colloids administered at the acute phase of shock (first 36 hours), in univariate analysis. The volumes of hyperoncotic albumin could not be evaluated because volumes are inconsistent with those of other fluids. Patients were classified according to the volume of artificial hyperoncotic colloids administered within the first 36 hours of resuscitation: small (<1 L), moderate (1-2 L) or high (> 2 L). The incidence of renal adverse event was associated to the volume of artificial hyperoncotic colloids (p=0.004). The volume of artificial hyperoncotic colloids was then tested in a multivariate model developed in the subgroup of patients resuscitated with artificial hyperoncotic colloids (table E3). After adjustment a volume of artificial hyperoncotic colloids over 2 liters within 36 hours remained significantly associated with a higher incidence of renal adverse events.
Because the volume of expanders administered could be a marker of severity, we used similar volume classification in patients who received hypooncotic fluids only. In univariate analysis we assessed the association with renal adverse event. The volume of hypooncotic fluids was not associated with the occurrence of renal adverse event (p=0.12) (Figure E1).
3 Figure E1: incidence of renal adverse events according to the volume of fluids administered in the subgroups of patients resuscitated using hypooncotic fluids only and of patients resuscitated using artificial hyperoncotic colloids.
4 Table E3. Results of Logistic Regression for renal adverse events occurrence in patients receiving artificial hyperoncotic colloids (n=401).
Analysis in Presence of Analysis in Presence of Covariates without Covariates with Propensity Propensity Score† Score‡
Risk Factor Odds Ratio (95% CI) P Value Odds Ratio (95% P Value CI)
Age, year 1.00 (0.98-1.02) 0.896 1.00 (0.98-1.05) 0.958
SAPS II , point 1.03 (1.01-1.05) 0.004 1.03 (1.01-1.05) 0.006
Number of organ dysfunction 0.82 (0.59-1.13) 0.216 0.83 (0.59-1.16) 0.275 <0.000 Previous renal dysfunction 8.00 (2.67-23.94) <0.0001 7.92 (2.64-23.71) 1 Acute respiratory distress syndrome 2.67 (1.05-6.77) 0.039 2.64 (1.04-6.72) 0.041
Volume of artificial hyperoncotic colloids within the first 36 hrs of resuscitation, liter
0.0 – 1.00 1.00 0.015 1.00 0.014
> 1.00 – 2.00 1.72 (0.91-3.23) 1.75 (0.92-3.33)
> 2.00 2.21 (1.12-4.36) 2.29 (1.14-4.61) Abbreviations: SAPS, simplified acute physiology score; CI, confidence interval. † The goodness-of-fit chi-square test was 10.94 (P=0.21). The area under ROC curve was 0.76. ‡ The goodness-of-fit chi-square test was 10.73 (P=0.22). The area under ROC curve was 0.76.
ANALYSIS ON THE IMPACT OF PLASMA INFUSION ON RENAL EVENT OCCURRENCE
In univariate analysis, plasma use was associated with occurrence of renal adverse events (50/169, 30% vs 61/337, 18%; p=0.003) but not with mortality (53/167, 32% vs 92/334, 28%; p=0.33). After adjustment, the use of plasma did not remain associated with worse renal outcome in patients receiving hyperoncotic colloids: OR 1.33 (95% CI: 0.77-2.28).
5