Experiment #2 / Unit 5 Molar Volume of Butane
Introduction: Avogadro believed that equal numbers of different gases will occupy the same volume if they are at the same conditions of temperature and pressure. Avogadro's findings worked for any number of molecules (as long as you were comparing equal numbers) and at any conditions (as long as each sample was at the same T and P). However, scientists like to use a standard point of reference and comparison. The most logical is to compare exactly one mole of every gas at standard temperature and pressure. This is the concept of molar volume whereby a mole of all gases will occupy 22.4 L of space at STP. We have already collected hydrogen and determined its molar volume. Now we will collect a much larger gas molecule and determine if it will occupy the same amount of space as hydrogen. The gas will be butane from a lighter and we will collect it in much the same way as the hydrogen and carry out similar kinds of measurements and calculations.
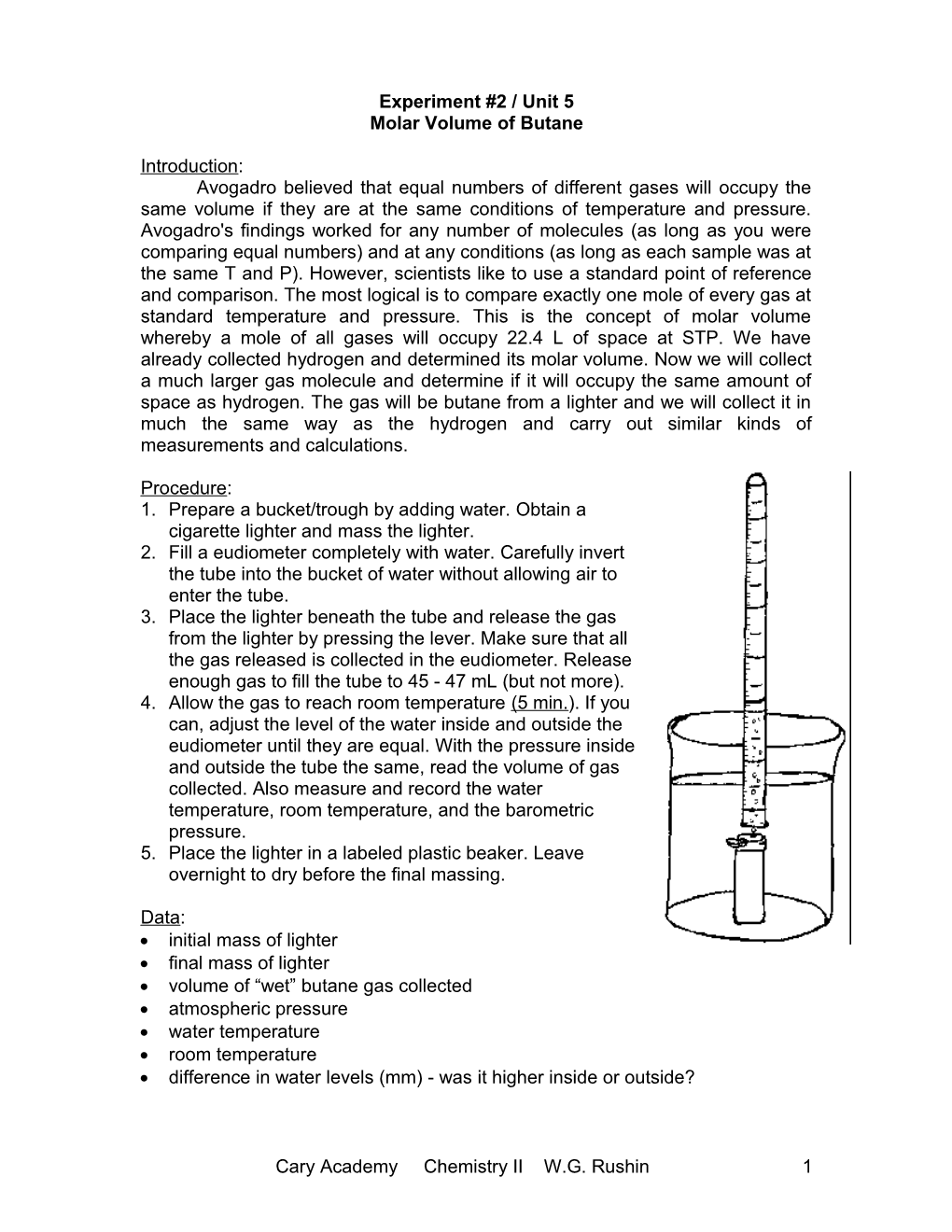
Procedure: 1. Prepare a bucket/trough by adding water. Obtain a cigarette lighter and mass the lighter. 2. Fill a eudiometer completely with water. Carefully invert the tube into the bucket of water without allowing air to enter the tube. 3. Place the lighter beneath the tube and release the gas from the lighter by pressing the lever. Make sure that all the gas released is collected in the eudiometer. Release enough gas to fill the tube to 45 - 47 mL (but not more). 4. Allow the gas to reach room temperature (5 min.). If you can, adjust the level of the water inside and outside the eudiometer until they are equal. With the pressure inside and outside the tube the same, read the volume of gas collected. Also measure and record the water temperature, room temperature, and the barometric pressure. 5. Place the lighter in a labeled plastic beaker. Leave overnight to dry before the final massing.
Data: initial mass of lighter final mass of lighter volume of “wet” butane gas collected atmospheric pressure water temperature room temperature difference in water levels (mm) - was it higher inside or outside?
Cary Academy Chemistry II W.G. Rushin 1 Calculations and Questions:
1. Write a balanced equation for a cigarette lighter flame (butane = C4H10). 2. Calculate the mass of butane gas used. 3. Calculate the moles of butane gas used. 4. Why are the molecules of butane inside the lighter condensed into a pool of liquid? Your answer should involve a Lewis structure, a VSEPR shape model, electronegativity, dipoles, polarity, and intermolecular forces. 5. What would happen to the liquid butane if you poured it out on the benchtop? Why? 6. How many molecules of CO2 would be produced if this butane were burned? 7. What was the partial pressure of the water vapor which contaminated the butane?
8. Calculate Pdiff.
9. Calculate the partial pressure of “dry” butane gas (PC4H10). 10.Convert the volume of butane to STP. 11.Calculate the molar volume (L/mol) of the butane at STP. 12.Calculate the percent error for your experiment based on your experimental molar volume. The accepted value is 22.4 L/mol at STP. 13.Why did I tell you to collect only 45-47 mL of butane and make sure you allowed it to reach room temperature? 14.Explain how a mole of butane gas that is 29 times larger in mass than hydrogen can occupy the same volume.
Lab Report #5.2: title page procedure sheet data calculations and questions
Cary Academy Chemistry II W.G. Rushin 2