Supplementary Material
Cell-laden Polymeric Microspheres for Biomedical Applications Wenyan Leong and Dong-An Wang Division of Bioengineering, School of Chemical & Biomedical Engineering, Nanyang Technological University, 70 Nanyang Drive, Singapore 637457 Corresponding author: Wang. D.-A. ([email protected])
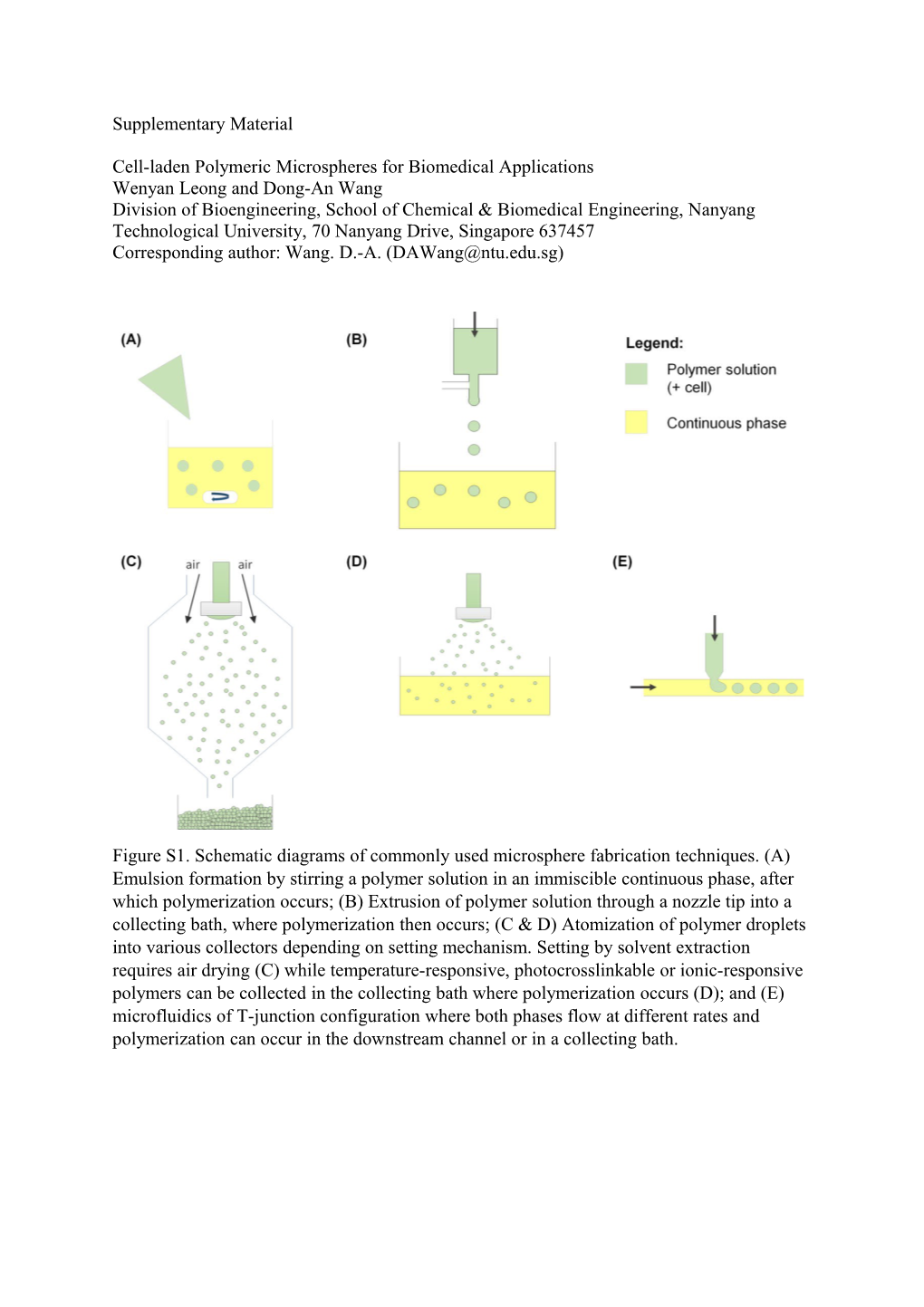
Figure S1. Schematic diagrams of commonly used microsphere fabrication techniques. (A) Emulsion formation by stirring a polymer solution in an immiscible continuous phase, after which polymerization occurs; (B) Extrusion of polymer solution through a nozzle tip into a collecting bath, where polymerization then occurs; (C & D) Atomization of polymer droplets into various collectors depending on setting mechanism. Setting by solvent extraction requires air drying (C) while temperature-responsive, photocrosslinkable or ionic-responsive polymers can be collected in the collecting bath where polymerization occurs (D); and (E) microfluidics of T-junction configuration where both phases flow at different rates and polymerization can occur in the downstream channel or in a collecting bath. Table S1 Properties of common polymers for microsphere fabrication
Polymers Type, source Common Reversible? Advantages Disadvantages Ref crosslinking mechanism Natural Typically - Biodegradable - Batch-to-batch polymers physical - Low toxicity variation crosslinked - Low cost, widely - Presence of impurities, available requires stringent - Typically mild purification crosslinking, allowing - Usually weak direct cell encapsulation mechanical properties, suitable for mimicking soft tissue only - Uncontrollable degradation, unstable
- Alginate Polysaccharide, Ionic: Yes. - Suitable for direct cell - Non-cell adhesive; [S1] brown algae Introduce Immersion in encapsulation - Susceptible to divalent ions for chelating agents - Mild crosslinking uncontrollable sol-gel transition (sodium citrate) - High water content dissolution in presence of monovalent ions - Agarose Polysaccharide, Temperature: No. - Suitable for direct cell - Non-cell adhesive [S2] seaweed Decrease encapsulation - Brittle temperature for - High water content sol-gel transition
- Collagen Protein, Self-assembly Yes. - Cell-adhesive - Allogenic or [S3, connective Enzymatic - ECM xenogeneic source, S4] tissue of animal degradation by - Good mechanical may induce host sources MMPs properties immune response - Suitable for direct cell when transplanted encapsulation
- Chitosan Polysaccharide, pH: Yes. - Cell-adhesive - Not able to encapsulate [S5, deacylation of increase pH for Enzymatic - Good mechanical cells directly; cells can S6] chitin sol-gel transition degradation by properties be seeded after lysozyme - Possess amino groups fabrication for functionalization - Degree of deacylation is not precise - Low hydrophilicity - Slow degradation
- Fibrin Protein, blood Self-assembly: Yes. - Cell-adhesive - Allogenic or [S7] plasma Fibrinogen Enzymatic - Highly compliant xenogeneic source, cleavage by degradation by - Suitable for direct cell may induce host thrombin to plasmin encapsulation immune response form fibrin when transplanted monomers, self- - Chance of viral assembly to transmission (prepared form fibrous from blood) network
- Gellan Polysaccharide, Ionic: Yes. Increase in - FDA-approved - Lack of cell-adhesive [S8, fermentation of Introduce temperature. - Good mechanical moieties; requires S9] Spinghomonas multivalent properties functionalization elodea cations for sol- - High acyl content gives gel transition; or compliant and elastic Temperature gels - Suitable for direct cell encapsulation
- Gelatin Protein, Temperature: Yes. - Cell-adhesive - Quick dissolution [S10, denatured Decrease Increase in - ECM-like under physiological S11] collagen temperature for temperature or - Suitable for direct cell temperature; require sol-gel transition enzymatic encapsulation covalent crosslinking degradation by MMPs
Synthetic Typically No. - Easily tunable - Lack of cell-adhesive polymers chemically Require mechanical and moieties; requires crosslinked modification chemical properties functionalization e.g. - Stable - Usually harsh photodegradable - Consistent quality (no/ fabrication conditions crosslinkers little batch-to-batch - Expensive variation) - Non-degradable/ toxic degradation products
- Poly(ethylen Polyester Covalent No - FDA approved; - Lack of cell-adhesive [S12, e glycol) biocompatible moieties S13] (PEG) - Suitable for direct cell encapsulation
- Poly(lactic- Polyester Solvent No - FDA approved; - Hydrophobic [S14- co-glycolic extraction/ biocompatible - Lack of cell-adhesive S16] acid) evaporation - Non-toxic moieties (PLGA) - Bioresorbable - - Poly(caprola Polyester Solvent No - FDA approved; - Lack of cell-adhesive [S17, ctone) (PCL) extraction/ biocompatible moieties S18] evaporation - Bioresorbable - Slower degradation than PLGA - Ease of blending with other polymers Table S2 Common microsphere fabrication techniques
Technique Principles Advantages Disadvantages Factors affecting microsphere size Example ref Emulsion - Two immiscible - No specialized - Large size - Size of vessel Collagen [S3], phases. equipment required distribution - Viscosities of phases Fibrin [S7], Dispersed phase - Scalable - Washing steps - Stirring rate Gelatin [S11], contains - Multiple emulsions required - Presence and concentration of PLGA [S16], polymer achievable - Batch-wise surfactant Chitosan [S19] solution. - High throughput process - Mechanical agitation/ stirring to break up polymer solution into droplets
Extrusion - Polymer - Uniformly sized - Specialized - Flow rate Chitosan [S5, solution is microspheres equipment - Viscosity of polymer solution S6], extruded - Continuous process required - Diameter of nozzle Alginate [S1, through a nozzle achievable - Low throughput S20] and fall into (1 bead at a time) collector
Atomization - Atomized - Small microspheres - Specialized - Flow rate PVA [S21], droplets fall into with narrow size equipment - Viscosity of polymer solution alginate [S22] a collector distribution required - Frequency - High throughput - Height of collector and air flow - Continuous process direction (for spray drying) achievable - Temperature - Scalable Microfluidics - Two immiscible - Uniformly sized - Washing steps - Flow rates Agarose [S2], phases flowing microspheres required - Viscosities of phases PEG [S23], continuously, - Easy to customize - Not scalable - Diameter of nozzle Poly(NIPAAm) with continuous - Small platform - Low throughput - Dimension of setup [S24] phase pinching - Continuous process (1 bead at a time) - Presence and concentration of off beads of achievable surfactant dispersed phase - Multiple emulsions (polymer achievable solution) Table S3 List of commercially available microcarriers, data obtained from respective companies. Unless stated as macroporous, the microcarriers are microporous.
Material Commercial name/ Density Coating/ Feature Mean Company (g/ cm3) Diameter (µm) Dextran Cytodex 1 1.03 - Positively charged 190 (GE Healthcare) Cytodex 3 1.04 - Porcine gelatin- 175 (GE Healthcare) coated - Gelatin Cultispher S 1.02-1.04 - High thermal 130-380 (Sigma Aldrich) stability - Macroporous Cultispher G 1.02-1.04 - Macroporous 130-380 (Sigma Aldrich)
Collagen Spherecol 1.02-1.03 - Human type I 125-212 (Advanced collagen-coated Biomatrix)
Cellulose Cytopore 1 1.1 - Positively charged 230 (GE Healthcare) - Macroporous; increases surface area Cytopore 2 1.1 - Highly positively 230 (GE Healthcare) charged - Macroporous - Polystyrene BB-MF 3092 1.02 - Porcine gelatin- 125-212 (SoloHill, Sigma) coated Synthemax II 1.026 - Collagen 125-212 (Corning) References S1 Chayosumrit, M., et al. (2010) Alginate microcapsule for propagation and directed differentiation of hESCs to definitive endoderm. Biomaterials 31, 505-514 S2 Kumachev, A., et al. (2011) High-throughput generation of hydrogel microbeads with varying elasticity for cell encapsulation. Biomaterials 32, 1477-1483 S3 Yao, L., et al. (2013) Collagen microsphere serving as a cell carrier supports oligodendrocyte progenitor cell growth and differentiation for neurite myelination in vitro. Stem Cell Res Ther 4, 1-8 S4 Cheng, H.W., et al. (2011) In vitro generation of an osteochondral interface from mesenchymal stem cell-collagen microspheres. Biomaterials 32, 1526-1535 S5 Skop, N.B., et al. (2013) Heparin crosslinked chitosan microspheres for the delivery of neural stem cells and growth factors for central nervous system repair. Acta Biomaterialia 9, 6834-6843 S6 Yang, Z., et al. (2014) Chitosan Microsphere Scaffold Tethered with RGD-Conjugated Poly(methacrylic acid) Brushes as Effective Carriers for the Endothelial Cells. Macromolecular bioscience 14, 1299-1311 S7 Xie, M.W., et al. (2013) Marrow-derived stromal cell delivery on fibrin microbeads can correct radiation-induced wound-healing deficits. The Journal of investigative dermatology 133, 553-561 S8 Pereira, D.R., et al. (2011) Development of gellan gum-based microparticles/hydrogel matrices for application in the intervertebral disc regeneration. Tissue engineering. Part C, Methods 17, 961-972 S9 Wang, C., et al. (2008) A novel gellan gel-based microcarrier for anchorage-dependent cell delivery. Acta Biomater 4, 1226-1234 S10 Lau, T.T., et al. (2012) Formation of model hepatocellular aggregates in a hydrogel scaffold using degradable genipin crosslinked gelatin microspheres as cell carriers. Biomedical materials (Bristol, England) 7, 065003 S11 Leong, W., et al. (2013) A temperature-cured dissolvable gelatin microsphere-based cell carrier for chondrocyte delivery in a hydrogel scaffolding system. Acta Biomaterialia 9, 6459-6467 S12 Allazetta, S., et al. (2013) Microfluidic synthesis of cell-type-specific artificial extracellular matrix hydrogels. Biomacromolecules 14, 1122-1131 S13 Olabisi, R.M., et al. (2010) Hydrogel Microsphere Encapsulation of a Cell-Based Gene Therapy System Increases Cell Survival of Injected Cells, Transgene Expression, and Bone Volume in a Model of Heterotopic Ossification. Tissue engineering. Part A 16, 3727-3736 S14 Jung, M.-S., et al. (2013) In vitro micro-mineralized tissue formation by the combinatory condition of adipose-derived stem cells, macroporous PLGA microspheres and a bioreactor. Macromolecular Research 22, 47-57 S15 Huang, C.C., et al. (2012) Injectable PLGA porous beads cellularized by hAFSCs for cellular cardiomyoplasty. Biomaterials 33, 4069-4077 S16 Bible, E., et al. (2009) The support of neural stem cells transplanted into stroke-induced brain cavities by PLGA particles. Biomaterials 30, 2985-2994 S17 Fujii, S., et al. (2013) Hydroxyapatite-coated poly(ϵ-caprolactone) microspheres fabricated via a Pickering emulsion route: effect of fabrication parameters on diameter and chemical composition. Composite Interfaces 20, 45-56 S18 Jang, T.-S., et al. (2012) Hollow porous poly(ε-caprolactone) microspheres by emulsion solvent extraction. Materials Letters 72, 157-159 S19 Fang, J., et al. (2014) Poly(l-glutamic acid)/chitosan polyelectrolyte complex porous microspheres as cell microcarriers for cartilage regeneration. Acta Biomaterialia 10, 276-288 S20 Spuch, C., et al. (2010) The effect of encapsulated VEGF-secreting cells on brain amyloid load and behavioral impairment in a mouse model of Alzheimer’s disease. Biomaterials 31, 5608-5618 S21 Young, C.J., et al. (2012) Combining submerged electrospray and UV photopolymerization for production of synthetic hydrogel microspheres for cell encapsulation. Biotechnology and bioengineering 109, 1561-1570 S22 Gurruchaga, H., et al. (2015) Cryopreservation of microencapsulated murine mesenchymal stem cells genetically engineered to secrete erythropoietin. International Journal of Pharmaceutics 485, 15-24 S23 Headen, D.M., et al. (2014) Microfluidic-Based Generation of Size-Controlled, Biofunctionalized Synthetic Polymer Microgels for Cell Encapsulation. Advanced Materials 26, 3003-3008 S24 Seiffert, S., et al. (2010) Janus Microgels Produced from Functional Precursor Polymers. Langmuir 26, 14842-14847