Equilibrium Constant Expressions
Recall:
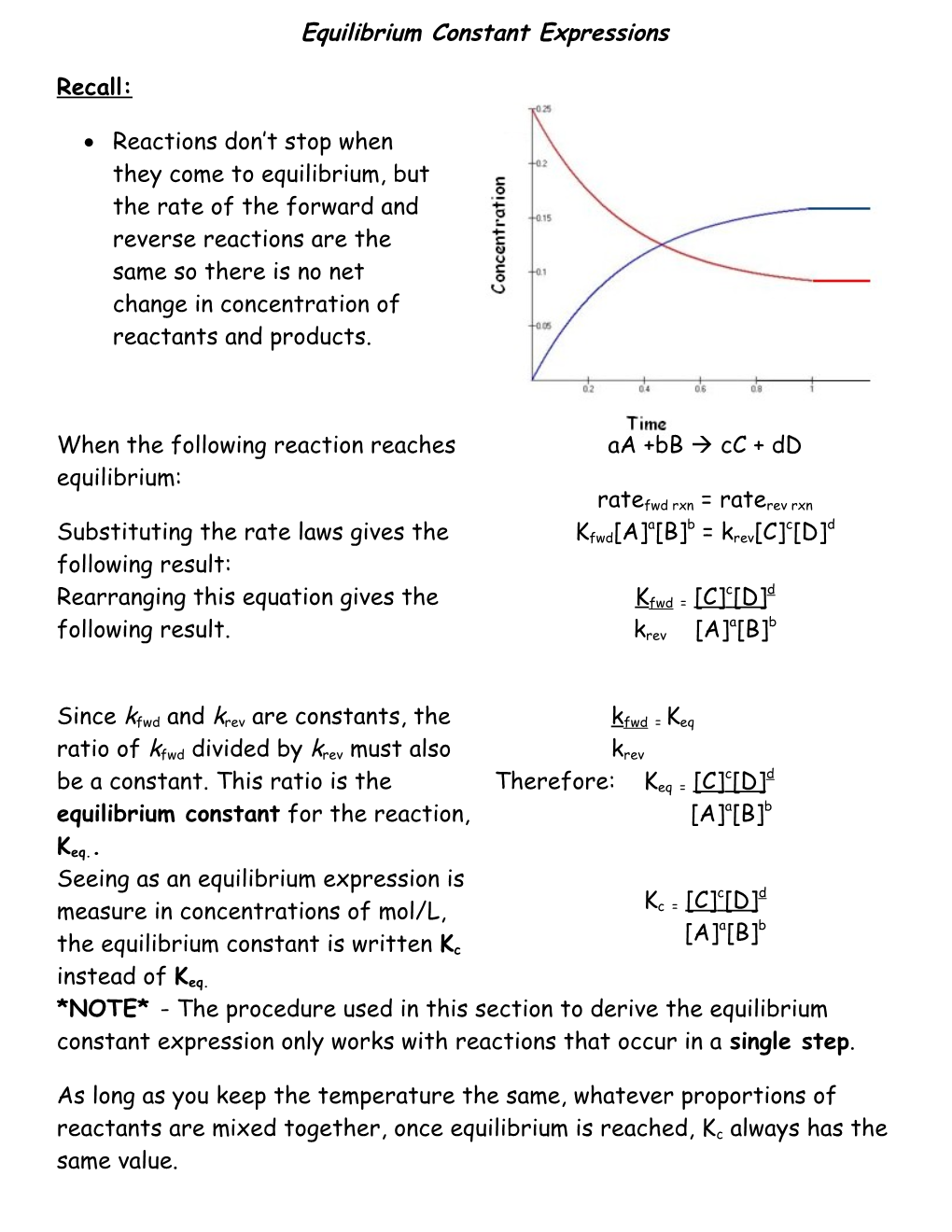
Reactions don’t stop when they come to equilibrium, but the rate of the forward and reverse reactions are the same so there is no net change in concentration of reactants and products.
When the following reaction reaches aA +bB cC + dD equilibrium: ratefwd rxn = raterev rxn a b c d Substituting the rate laws gives the Kfwd[A] [B] = krev[C] [D] following result: c d Rearranging this equation gives the Kfwd = [C] [D] a b following result. krev [A] [B]
Since kfwd and krev are constants, the kfwd = Keq ratio of kfwd divided by krev must also krev c d be a constant. This ratio is the Therefore: Keq = [C] [D] equilibrium constant for the reaction, [A]a[B]b
Keq.. Seeing as an equilibrium expression is c d measure in concentrations of mol/L, Kc = [C] [D] [A]a[B]b the equilibrium constant is written Kc instead of Keq. *NOTE* - The procedure used in this section to derive the equilibrium constant expression only works with reactions that occur in a single step.
As long as you keep the temperature the same, whatever proportions of reactants are mixed together, once equilibrium is reached, Kc always has the same value. Questions
1. Write equilibrium constant expressions for the following reactions.
2. Calculate the value of the equilibrium constant, Keq , for the system shown:
CO2(g) + H2(g) CO(g) + H2O(g)
If 0.1908 moles of CO2, 0.0908 moles of H2, 0.0092 moles of CO, and 0.0092 moles of H2O vapor were present in a 2.00 L reaction vessel were present at equilibrium.
What does the value of the equilibrium constant indicate?
Keq > 1 At equilibrium there is more product than reactant – aka product favoured. If K is greater than 1010 the reaction is regarded as going to completion.
Keq < 1 At equilibrium there is more reactant than product – aka reactant favoured. If K is less than 10-10 the reaction is regarded as not taking place at all.
Keq = 1 At equilibrium there is an equal amount of product and reactant
Note: Can we change the concentration of solids and liquids?
No! Therefore do NOT include them in the equilibrium constant expression
E.g. Write the Keq expression for:
a) The combustion of pentane b) Dissolution of calcium chloride
C5H12(l) + 8 O2(g) 5 CO2(g) + 6 H2O(l)
2+ - CaCl2(s) Ca (aq) + 2 Cl (aq)