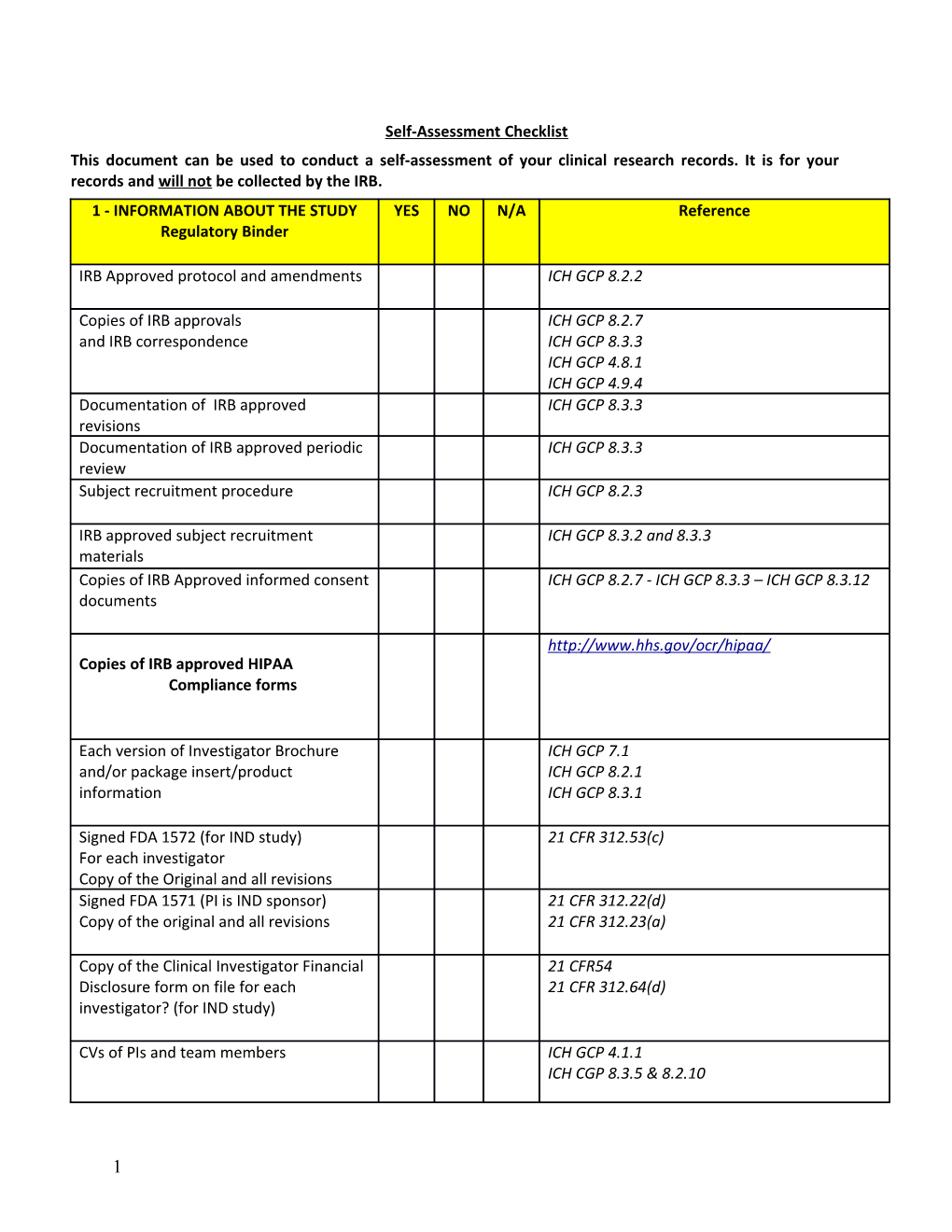
Self-Assessment Checklist This document can be used to conduct a self-assessment of your clinical research records. It is for your records and will not be collected by the IRB. 1 - INFORMATION ABOUT THE STUDY YES NO N/A Reference Regulatory Binder
IRB Approved protocol and amendments ICH GCP 8.2.2
Copies of IRB approvals ICH GCP 8.2.7 and IRB correspondence ICH GCP 8.3.3 ICH GCP 4.8.1 ICH GCP 4.9.4 Documentation of IRB approved ICH GCP 8.3.3 revisions Documentation of IRB approved periodic ICH GCP 8.3.3 review Subject recruitment procedure ICH GCP 8.2.3
IRB approved subject recruitment ICH GCP 8.3.2 and 8.3.3 materials Copies of IRB Approved informed consent ICH GCP 8.2.7 - ICH GCP 8.3.3 – ICH GCP 8.3.12 documents
http://www.hhs.gov/ocr/hipaa/ Copies of IRB approved HIPAA Compliance forms
Each version of Investigator Brochure ICH GCP 7.1 and/or package insert/product ICH GCP 8.2.1 information ICH GCP 8.3.1
Signed FDA 1572 (for IND study) 21 CFR 312.53(c) For each investigator Copy of the Original and all revisions Signed FDA 1571 (PI is IND sponsor) 21 CFR 312.22(d) Copy of the original and all revisions 21 CFR 312.23(a)
Copy of the Clinical Investigator Financial 21 CFR54 Disclosure form on file for each 21 CFR 312.64(d) investigator? (for IND study)
CVs of PIs and team members ICH GCP 4.1.1 ICH CGP 8.3.5 & 8.2.10
1 Updated Research Personnel List on file ICH GCP 4.1.5
Research staff have completed NU ICH GCP 2.8 mandatory trainings
The staff signature log, including ICH GCP 4.1.5 delegation of responsibility, is completed ICH GCP 8.3.24
Case report form or data collection page ICH GCP 8.3.14 templates
Adverse Event report forms and 21 CFR 312.32 - 21 CFR 312.64(B) instructions for sponsor and IRB reporting 21 CFR 312.66 - ICH GCP 1.50 ICH GCP 4.11.1
Copy of normal laboratory values ICH GCP 8.2.11 and 8.3.6 Laboratory certification on file (if not ICH 8.2.12 and 8.3.7 using hospital lab) performing work toward the completion of your study. IND Safety reports
Subject identification log is completed ICH GCP 8.3.21
Subject enrollment log is completed ICH GCP 8.3.22
Study Drug dispensing log is completed ICH GCP 8.3.23
2 - INFORMATION ABOUT THE SUBJECT YES NO N/A Reference
Informed Consent Form
The appropriate consent form is 21 CFR 50.27(a) used and signed prior enrollment of the ICH GCP 4.8.8 – 8.3-12 subject Subjects receive a copy of the signed 21 CFR 50.27 (a) consent form ICH GCP 4.8.11 This receipt is documented HIPAA Compliance
The Appropriate HIPAA Authorization is signed by the subject
The subject receives a copy of the signed HIPAA authorization
2 Eligibility
There is a list of Inclusion/Exclusion criteria completed for the subject
The list includes signature/initials of the person obtaining the information? The subject is eligible according to the ICH GCP 8.3.14 protocol Study Treatment/Outcome Response
ICH GCP 4.5.1 Study visits were scheduled in accordance with the study protocol ICH GCP 2.7 Study treatment was prescribed by the ICH GCP 4.6.5 authorized investigator according to the protocol
Study treatment monitoring and follow- up procedures were performed according to the protocol ICH GCP 8.3.14 Study treatment was accurately reported on the case report forms when compared to the source document The subject completed the study and was evaluable for response Adverse Events were properly reported to the sponsor/IRB
Drug Accountability
The subject’s treatment doses are 21 CFR 312.62 (a) accurately reported on the Dispensing ICH GCP 4.6.3 Log ICH GCP 4.6.3
3