Supporting Information Development of a Dielectric Barrier Discharge Ion Source for Ambient Mass Spectrometry
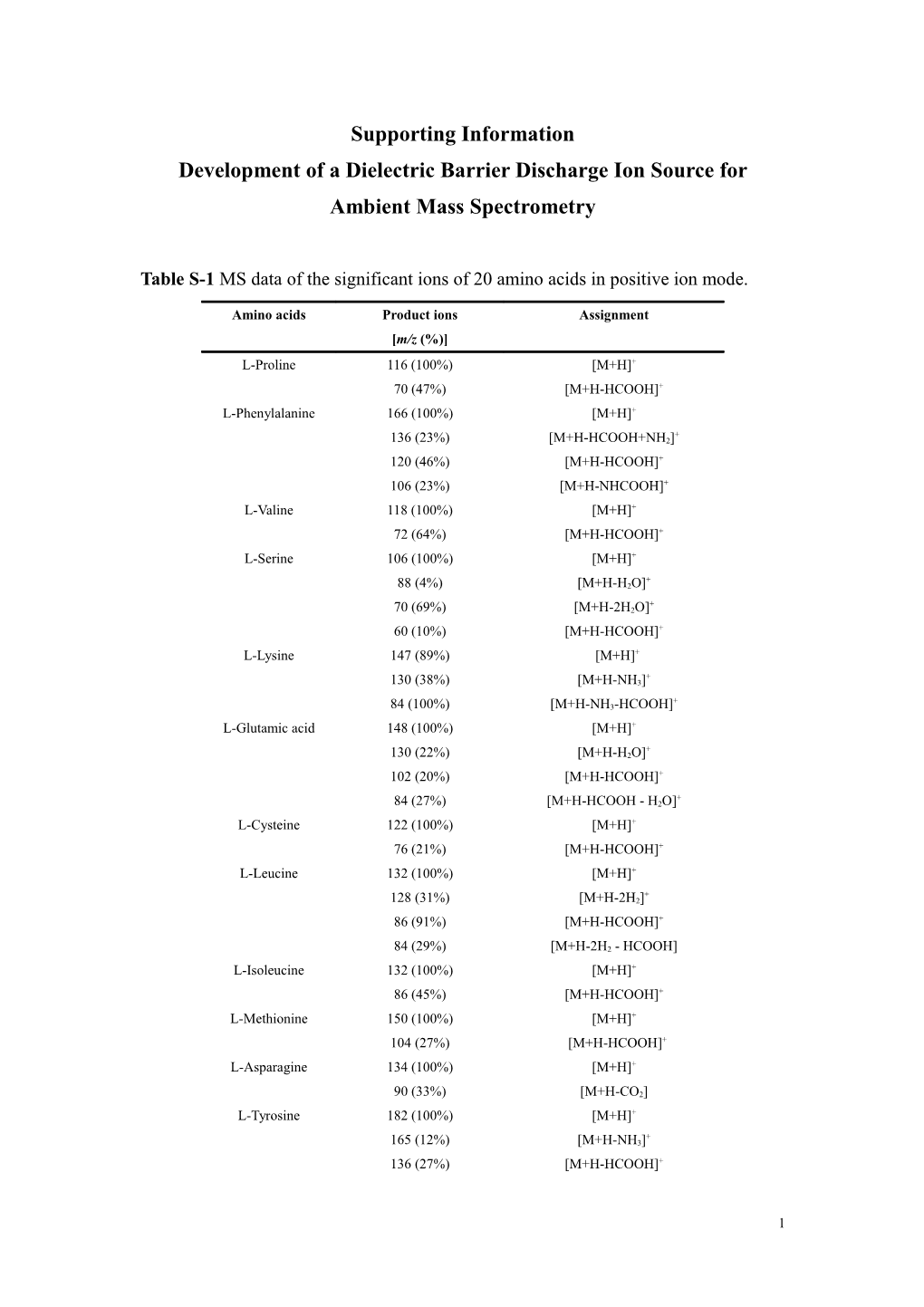
Table S-1 MS data of the significant ions of 20 amino acids in positive ion mode.
Amino acids Product ions Assignment [m/z (%)] L-Proline 116 (100%) [M+H]+ 70 (47%) [M+H-HCOOH]+ L-Phenylalanine 166 (100%) [M+H]+
+ 136 (23%) [M+H-HCOOH+NH2] 120 (46%) [M+H-HCOOH]+ 106 (23%) [M+H-NHCOOH]+ L-Valine 118 (100%) [M+H]+ 72 (64%) [M+H-HCOOH]+ L-Serine 106 (100%) [M+H]+
+ 88 (4%) [M+H-H2O]
+ 70 (69%) [M+H-2H2O] 60 (10%) [M+H-HCOOH]+ L-Lysine 147 (89%) [M+H]+
+ 130 (38%) [M+H-NH3]
+ 84 (100%) [M+H-NH3-HCOOH] L-Glutamic acid 148 (100%) [M+H]+
+ 130 (22%) [M+H-H2O] 102 (20%) [M+H-HCOOH]+
+ 84 (27%) [M+H-HCOOH - H2O] L-Cysteine 122 (100%) [M+H]+ 76 (21%) [M+H-HCOOH]+ L-Leucine 132 (100%) [M+H]+
+ 128 (31%) [M+H-2H2] 86 (91%) [M+H-HCOOH]+
84 (29%) [M+H-2H2 - HCOOH] L-Isoleucine 132 (100%) [M+H]+ 86 (45%) [M+H-HCOOH]+ L-Methionine 150 (100%) [M+H]+ 104 (27%) [M+H-HCOOH]+ L-Asparagine 134 (100%) [M+H]+
90 (33%) [M+H-CO2] L-Tyrosine 182 (100%) [M+H]+
+ 165 (12%) [M+H-NH3] 136 (27%) [M+H-HCOOH]+
1 + 111 (28%) [M-CH2NH2CHCOOH+H2O] L-Glycine 76 (100%) [M+H]+ L-Tryptophan 205 (100%) [M+H]+
+ 188 (89%) [M+H-NH3] 159 (9%) [M+H-HCOOH]+
+ 146 (13%) [M+H-CH2COOH]
+ 130 (11%) [M+H-CH2NH2COOH] L-Aspartic acid 134 (100%) [M+H]+
+ 116 (13%) [M+H-H2O]
+ 90 (30%) [M+H-CO2] L-Histidine 156 (100%) [M+H]+
+ 112 (71%) [M+H-CO2]
+ 95 (69%) [M+H-NH2COOH]
+ 83 (86%) [M-NH2CHCOOH+H2]
+ L-Arginine* 116 (100%) [M+H-HNC(NH2)2]
+ 71 (89%) [M+H-HNC(NH2)2- COOH] L-Glutamine 147 (100%) [M+H]+
+ 130 (78%) [M+H-NH3]
+ 102 (25%) [M+H-NH3-H2O]
+ 84 (94%) [M+H-NH3-HCOOH] L-Alanine 90 (100%) [M+H]+ 44 (67%) [M+H-HCOOH]+ L-Threonine 120 (100%) [M+H]+
+ 102 (9%) [M+H-H2O] 74 (24%) [M+H-HCOOH]+
* An exception that has not gotten ion of [M+H]+
2 90 100000 90 100000 a b 80000 80000 y
t 60000 y i
t 60000 i s s n n e t e t n I n 40000 I 40000
70 20000 20000 116 70 116
0 0 40 60 80 100 120 140 40 60 80 100 120 140 m/z m/z
120000 90 70 c 100000
80000 116 y t i
s 60000 n e
d -HCOOH n I 40000 72 -H O 2 20000 -CO 2
0 40 60 80 100 120 140 m/z
Figure S-1 The spectra of L-alanine (Mr 89) obtained by the different power of 5 W
(a), 18 W (b) and 30 W (c) at same integral time.
3 50000 76 a 30000 76 40000 b 24000
30000
y 18000 y t t i i s s n n e e t 20000 t n n I 12000 I
10000 6000
0 0 40 50 60 70 80 90 40 50 60 70 80 90 m/z m/z
76 80000 c
60000 y t i s n 40000 e t n I
20000
0 40 50 60 70 80 90 m/z
Figure S-2 The spectra of L-glycine (Mr 75) obtained by the gases of argon (a), air (b) and helium (c). The gas speed was 12 m/s. The L-glycine samples were deposited on the surface of filter paper.
4 70 1600000 116 1200000 116 b 1000000 a 1200000 800000 y t y i t 70 i s s 600000 n 800000 n e t e t n I n I 400000 -HCOOH 400000 -HCOOH 200000
0 0 40 60 80 100 120 140 40 60 80 100 120 140 m/z m/z
2000000 70 2000000 116
70 c 1500000 116 d 1500000 y y t t i i s s 1000000 n n
1000000 e e t t n n I I
500000 500000 -HCOOH -HCOOH
0 0 40 60 80 100 120 140 40 60 80 100 120 140 m/z m/z
Figure S-3 The spectra of L-proline (Mr 115) obtained at the helium speed of 12 m/s
(a), 24 m/s (b), 36 m/s (c) and 48 m/s (d). The L-proline samples were deposited on the surface of filter paper.
5 100 148 100 148 A B e e c c n n a a 130 d d n n u u 50 84
b 50 b A A
e e v v i i t 84 t a a 102 l 130 l e 102 -H O e R 2 R -H O 2 HCOOH 0 0 40 60 80 100 120 140 160 40 60 80 100 120 140 160 m/z m/z
Figure S-4 The spectra of L-Glutamic acid (A) and a commercial monosodium glutamate (B).
6