Initial Check for Received IRB Applications
This document describes the process used for initial checks of received IRB applications. To expedite review, please email the table shown in #3 below to [email protected]
1. Check whether the student’s research proposal/methodology approved by the Doctoral Program Director (or Designee). (Approval is needed before proceeding )
2. Check if student is enrolled in the current session at Trident. (Enrollment required before proceeding )
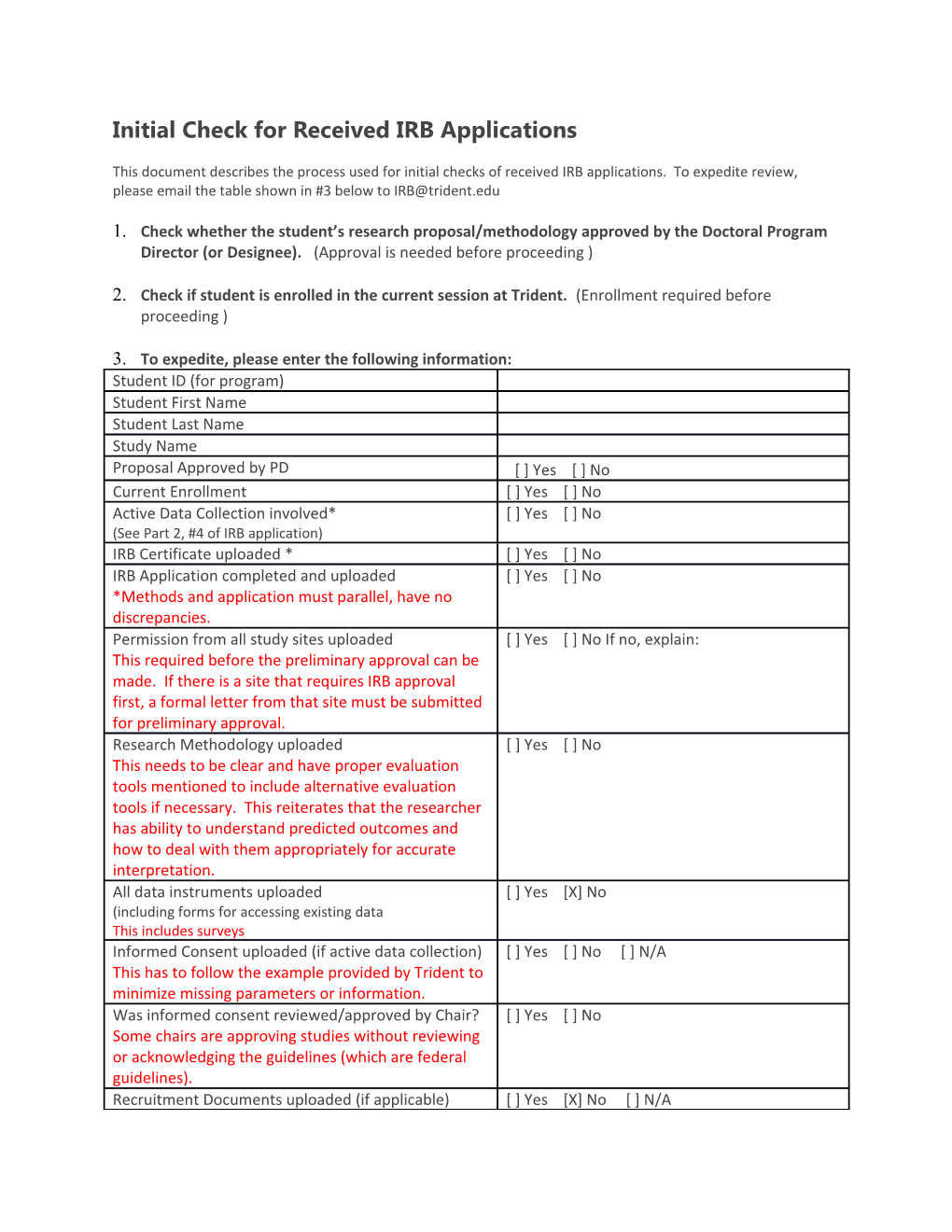
3. To expedite, please enter the following information: Student ID (for program) Student First Name Student Last Name Study Name Proposal Approved by PD [ ] Yes [ ] No Current Enrollment [ ] Yes [ ] No Active Data Collection involved* [ ] Yes [ ] No (See Part 2, #4 of IRB application) IRB Certificate uploaded * [ ] Yes [ ] No IRB Application completed and uploaded [ ] Yes [ ] No *Methods and application must parallel, have no discrepancies. Permission from all study sites uploaded [ ] Yes [ ] No If no, explain: This required before the preliminary approval can be made. If there is a site that requires IRB approval first, a formal letter from that site must be submitted for preliminary approval. Research Methodology uploaded [ ] Yes [ ] No This needs to be clear and have proper evaluation tools mentioned to include alternative evaluation tools if necessary. This reiterates that the researcher has ability to understand predicted outcomes and how to deal with them appropriately for accurate interpretation. All data instruments uploaded [ ] Yes [X] No (including forms for accessing existing data This includes surveys Informed Consent uploaded (if active data collection) [ ] Yes [ ] No [ ] N/A This has to follow the example provided by Trident to minimize missing parameters or information. Was informed consent reviewed/approved by Chair? [ ] Yes [ ] No Some chairs are approving studies without reviewing or acknowledging the guidelines (which are federal guidelines). Recruitment Documents uploaded (if applicable) [ ] Yes [X] No [ ] N/A Does study involve a vulnerable population? [ ] Yes [ ] No (See Part 3, #3 of IRB application) Access to Identifiers Does IRB application indicate identifiers may be [ ] Yes [ ] No accessed? (See Part 5 of IRB application) Do the data instruments contain questions asking for [ ] Yes [ ] No identifiers? Does the methodology (e.g. data collection section) [ ] Yes [ ] No indicate identifiers may be accessed? Does the informed consent (e.g. confidentiality [ ] Yes [ ] No section) indicate identifiers may be accessed? (For example, the term “anonymous implies that identifiers will not be collected. The term “confidential” implies that identifiers may be collected.) Will there be any crossmatching of data? [ ] Yes [ ] No (e.g. confirmation is needed if two or more data sources obtained from same participants) *See definitions below Some students are under the impression that the IRB just look for identity protection and that they IRB has no right to offer or expect methodical changes to ensure proper collection, evaluation, analysis and interpretation of human subject data. They actually have the right to question anything that would impede the aforemented. Hopefully, we can clear this up to maintain a positive forward flow.
DEFINITIONS
Active Data Collection: Refers to data collection that does not involve previously existing data (e.g. surveys, interviews, specimen collection, etc.)
IRB Certificate:
Personal Identifiers: Information that can identify an individual (such as name, phone, SSN, student identifier, etc.)
Vulnerable Population: Populations that require special treatment to safeguard well-being, such as children, prisoners, pregnant women, or handicapped or mentally disabled persons