Name ______Period ______Date ______
Atomic Structure & Periodic Table Study Guide 8th Grade Science
Test Date: Friday, Oct. 21
Parts of an Atom
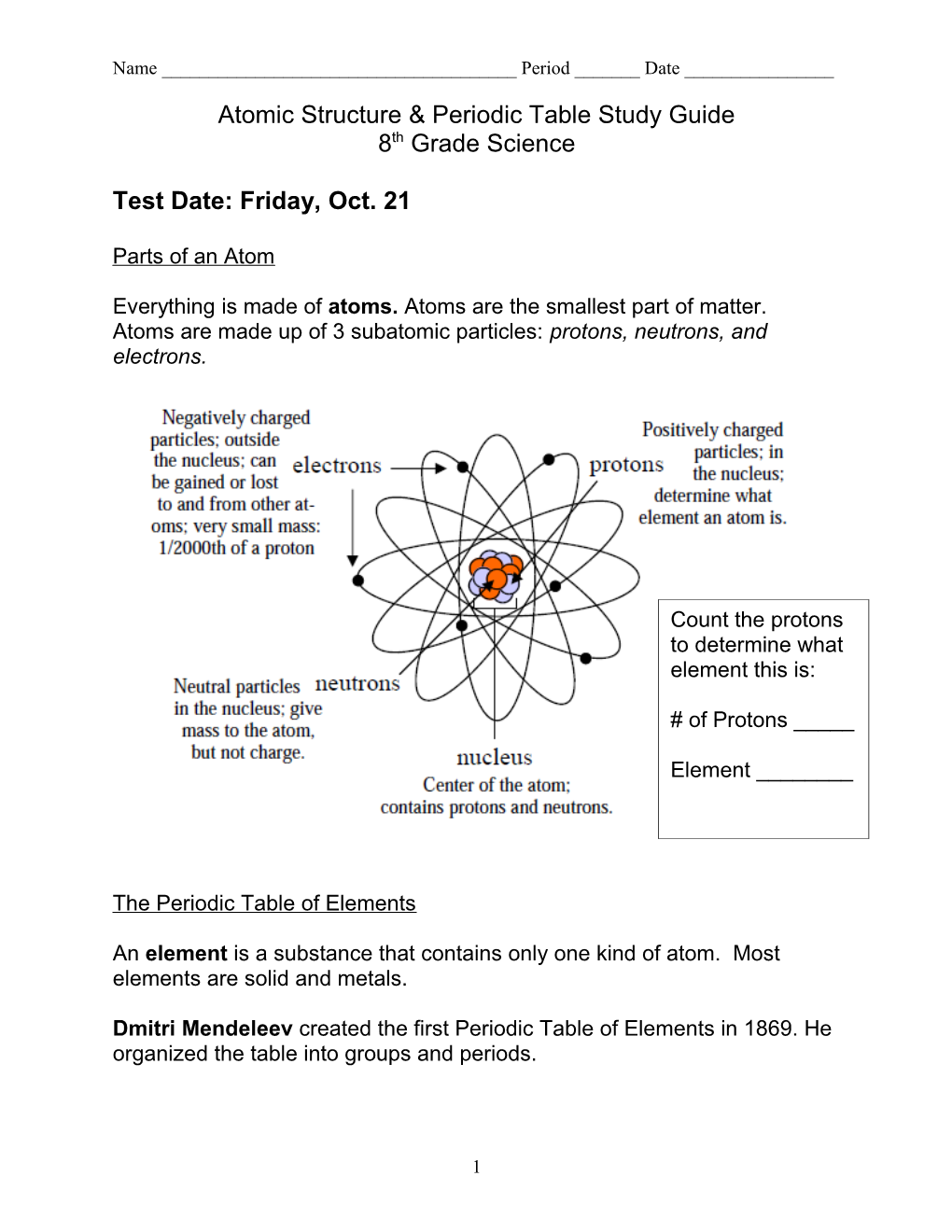
Everything is made of atoms. Atoms are the smallest part of matter. Atoms are made up of 3 subatomic particles: protons, neutrons, and electrons.
Count the protons to determine what element this is:
# of Protons _____
Element ______
The Periodic Table of Elements
An element is a substance that contains only one kind of atom. Most elements are solid and metals.
Dmitri Mendeleev created the first Periodic Table of Elements in 1869. He organized the table into groups and periods.
1 Name ______Period ______Date ______
Periods: Rows on the Periodic Table; each element in that row has the same number of energy levels (electron shells). Example: Period 1 (H and He) have only 1 energy level. There are 7 periods on the Periodic Table of Elements.
Groups or Families: Vertical columns on the Periodic Table; each element in the same group has similar physical properties (how it looks) and chemical properties (how it reacts with other elements). This happens because each element in that family or group has the same number of electrons in the outside energy level, which is known as the element’s valence electrons. There are 18 groups on the Periodic Table.
2 Name ______Period ______Date ______
Reactivity: Metals on the left side of the Periodic Table in Group 1 are very reactive (sometimes violently) when mixed with other elements. As you move to the right, through the metals, the elements become less reactive. Nonmetals have the opposite trend. Nonmetals are most reactive in Group 17, because they only need one valence electron to fill their outer shell. The Noble Gases, Group 18, are the most stable elements, because their energy levels are full.
Which element is the most reactive? a. Lithium b. Boron c. Carbon d. Neon 3 Name ______Period ______Date ______
Vocabulary Review
1. Proton a. particles with no charge that exists in the 2. Neutron nucleus of most atoms.
3. Electron b. center of the atom, contains most of the atom’s mass. 4. Nucleus c. total number of protons and neutrons in 5. Atom the nucleus of an atom.
6. Atomic Number d. number of protons in an atom; also the way the elements are numbered.
7. Atomic Mass e. electrons in the outermost energy level.
8. Energy Level f. Positively charged particle in the nucleus of the atom. Determines the element. 9. Valence Electrons g. Negative particles that spin around the 10. Element nucleus.
h. Neutral particles in the nucleus.
i. The space around the nucleus where electrons are held.
j. A substance that contains only one kind of atom
4 Name ______Period ______Date ______
5