Chemistry 130 Oregon State University Worksheet 3b Notes Dr. Richard Nafshun
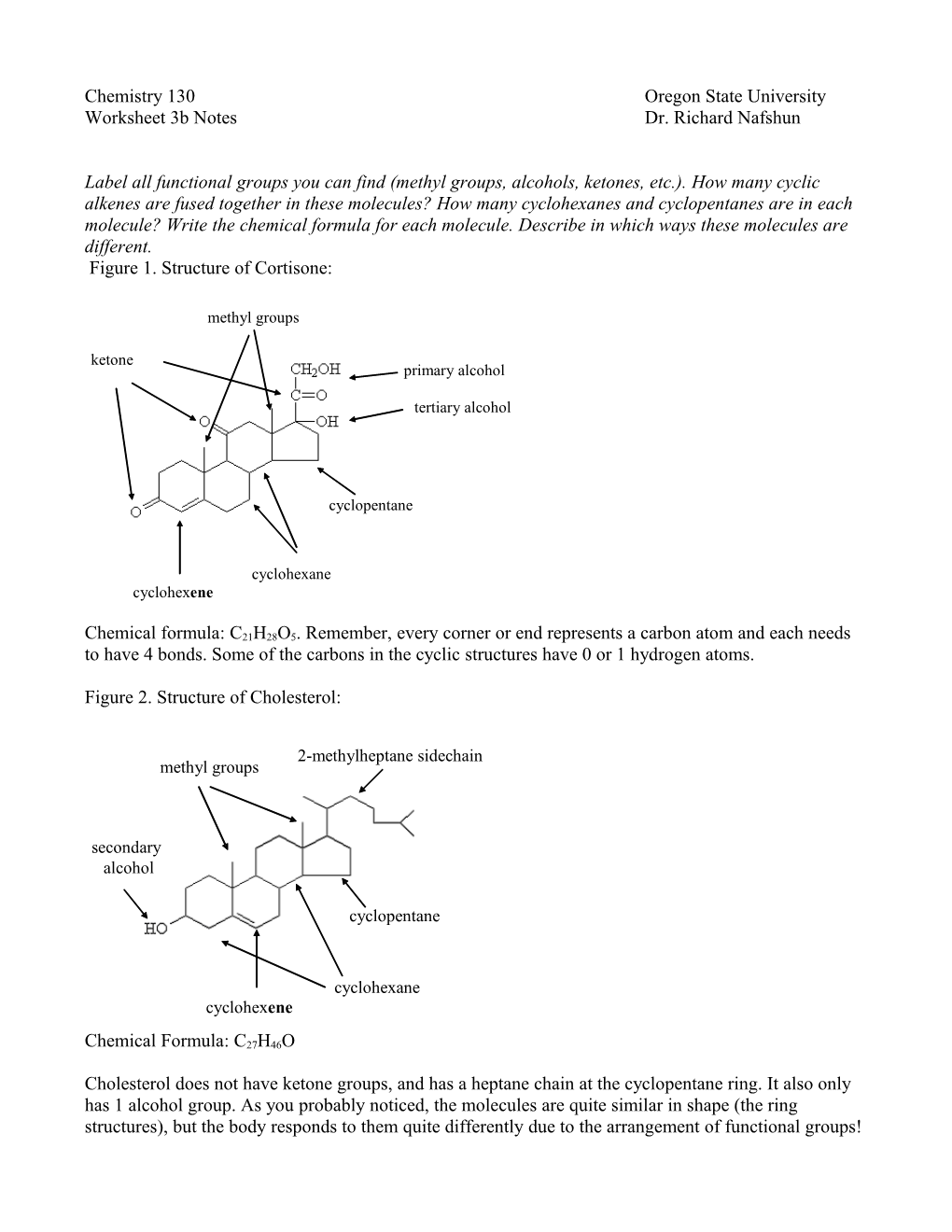
Label all functional groups you can find (methyl groups, alcohols, ketones, etc.). How many cyclic alkenes are fused together in these molecules? How many cyclohexanes and cyclopentanes are in each molecule? Write the chemical formula for each molecule. Describe in which ways these molecules are different. Figure 1. Structure of Cortisone:
methyl groups ketone primary alcohol
tertiary alcohol
cyclopentane
cyclohexane cyclohexene
Chemical formula: C21H28O5. Remember, every corner or end represents a carbon atom and each needs to have 4 bonds. Some of the carbons in the cyclic structures have 0 or 1 hydrogen atoms.
Figure 2. Structure of Cholesterol:
2-methylheptane sidechain methyl groups
secondary alcohol
cyclopentane
cyclohexane cyclohexene
Chemical Formula: C27H46O
Cholesterol does not have ketone groups, and has a heptane chain at the cyclopentane ring. It also only has 1 alcohol group. As you probably noticed, the molecules are quite similar in shape (the ring structures), but the body responds to them quite differently due to the arrangement of functional groups!