Introduction to Organic Synthesis
CM3001 Dr. Alan Ford (Lab 415) text: Willis & Wills Organic Synthesis (OUP)
To state the obvious:
Synthesis is the process of making a desired compound using chemical reactions. More often than not, more than one step is involved.
The importance of synthesis
1. Total synthesis of interesting and/or useful natural products 2. Industrially important compounds 3. compounds of theoretical interest 4. structure proof 5. development of new synthetic methodology 6. importance to other areas of science and technology
Examples
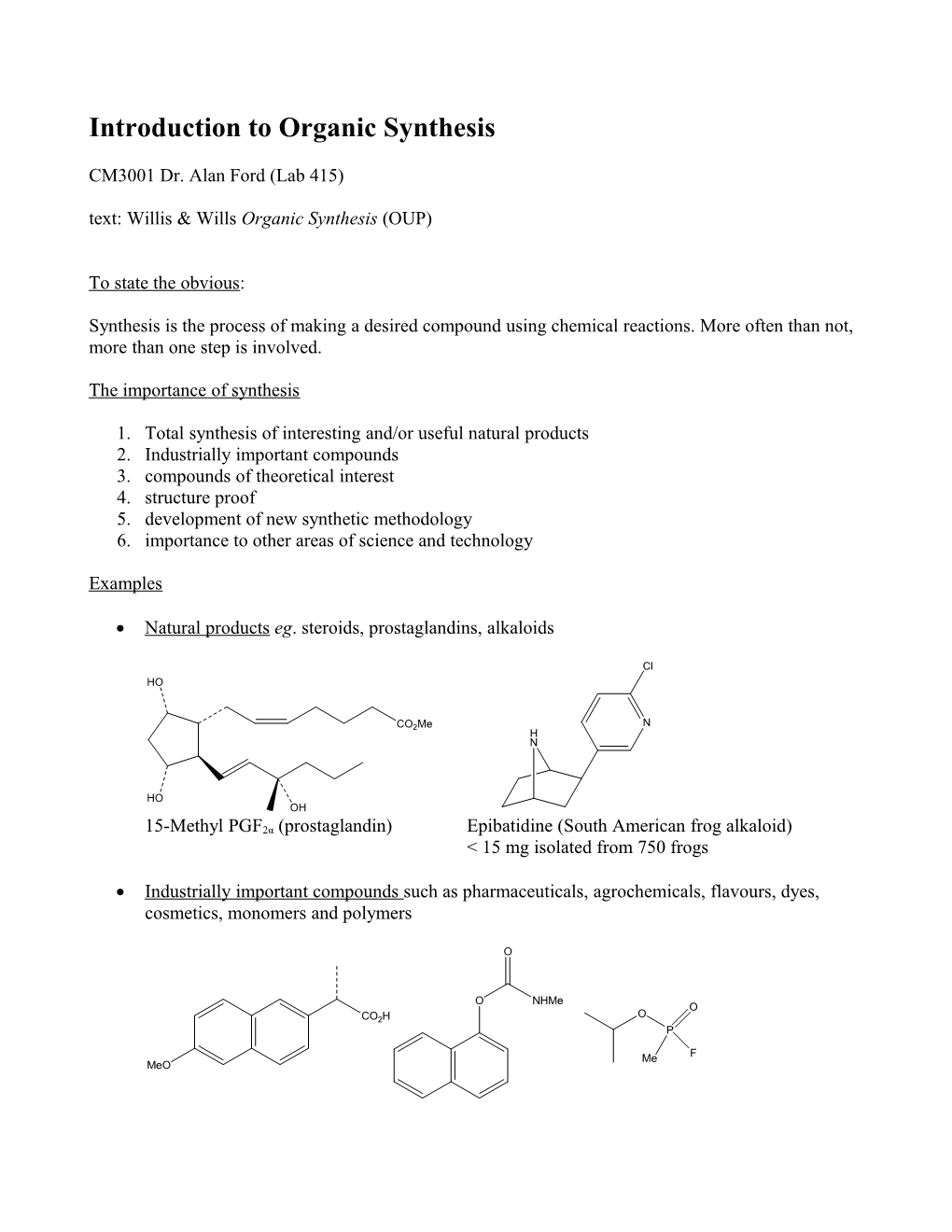
Natural products eg. steroids, prostaglandins, alkaloids
Cl HO
CO2Me N H N
HO OH
15-Methyl PGF2α (prostaglandin) Epibatidine (South American frog alkaloid) < 15 mg isolated from 750 frogs
Industrially important compounds such as pharmaceuticals, agrochemicals, flavours, dyes, cosmetics, monomers and polymers
O
O NHMe O O CO2H P
Me F MeO Naproxen (painkiller) Carbaryl (insecticide) Sarin (nerve gas)
CHO O O
O NHMe O OMe Isobutavan Methylenedioxymethamphetamine, MDMA (smells of mint chocolate) (Ecstasy)
H N CN O
N H
n O "5 CB" (liquid crystal) Kevlar (fancy polymer)
Theoretically interesting molecules
Cubane meta para Cyclophane
Structure proof While spectroscopy and crystallography are used to determine molecular structures, unambiguous total synthesis is still important
OH
H N
NH
MeO
Br S-(+)-Chelonin B (marine sponge alkaloid)
New methodology New ways to make molecules, improvement of existing ways, ways of doing what was previously impossible
2 Science and Technology Materials with special applications; molecular switches, non-linear optics, nanotechnology
Basic Steps of Solving Synthetic Problems
1) Choice of TARGET MOLECULE (TM)
2) Consideration of applicable synthetic methodology
3) Design of synthetic pathway
4) Execution of the synthesis
—these steps are highly interactive
Approaching the design of a synthesis (Part One)
For simple molecules it can be obvious just by looking at the target structure, for example:
Br
Cyclohexyl bromide
Bromoalkanes are available from alkenes or from alcohols
Br HBr
OH Br
PBr3
CO2Me
Methyl benzoate
Esters are available from carboxylic acids by reaction with alcohols; benzoic acid is available from toluene
CO Me CO2H 2 KMnO4 MeOH
H2SO4
3 cis-3-octene cis-Alkenes can be selectively prepared by partial reduction of alkynes; alkynes are accessible via acetylide chemistry.
NaNH2 H NaNH2
EtBr H Br H2 Lindlar's catalyst
Approaching the design of a synthesis (Part Two)
For more complex molecules, it helps to have a formalised, logic-centred approach; RETROSYNTHETIC ANALYSIS
Retrosynthetic analysis is the process of working backwards from the target molecule to progressively simpler molecules by means of DISCONNECTIONS and/or FUNCTIONAL GROUP INTERCONVERSIONS that correspond to known reactions. When you've got to a simple enough starting material (like something you can buy [and usually is cheap]) then the synthetic plan is simply the reverse of the analysis. The design of a synthesis needs to take into account some important factors. 1) it has to actually work 2) in general, it should be as short as possible 3) each step should be efficient 4) side products (if formed) and impurities (there always are) should be easily separable from the desired product 5) environmental issues may be relevant 6) there's more than one way to skin a cat
4 Example retrosynthetic analysis
Target molecule: OH
DISCONNECT A B
OH OH
SYNTHONS SYNTHONS
O
REAGENTS REAGENTS ? ? PhMgBr H therefore the target molecule could be synthesised as follows:
OH i) Mg/Et O Br 2
ii) CHO
What is a synthon?
When we disconnect a bond in the target molecule, we are imagining a pair of charged fragments that we could stick together, like Lego® bricks, to make the molecule we want. These imaginary charged species are called SYNTHONS. When you can think of a chemical with polarity that matches the synthon, you can consider that a SYNTHETIC EQUIVALENT of the synthon. Thus,
OH O
R H ≡ R H an aldehyde is a synthetic equivalent for the above synthon. There can be more than one synthetic equivalent for a given synthon, but if you can't think of one...try a different disconnection.
5 Always consider alternative strategies.
OH
DISCONNECT A B
OH OH
SYNTHONS SYNTHONS
BrMg Br Synthetic Synthetic PhCHO ? equivalents equivalents
a second possible synthesis:
OH
Br i) Mg/Et2O Ph ii) PhCHO
Similarly
OH OH
Ph Ph
O
BrMg Ph thus a third possible synthesis is
OH O BrMg
Ph Ph
6 Besides disconnections, we can also consider functional group interconversion. Our target molecule is a secondary alcohol, which could be prepared by reduction of a ketone. This is represented as follows:
O OH FGI Ph Ph
DISCONNECT
O O
Ph Br Ph (as enolate)
synthesis number four
O O
i) base LiAlH4 T.M. Ph Ph
ii) Br
Analysis number five:
O O
Ph Ph
O
LiCu( )2 Ph
Synthesis number five:
O O t-Bu CuLi NaBH4 2 T.M. Ph Ph
Disconnecting heteroatoms can also be a good idea:
7 OH OH "H2O"
Ph Ph Ph
6th approach:
OH
i) Hg(OAc)2 Ph Ph ii) NaBH4
There are other possibilities, but let's not bother with any more.
How do you choose which method?
Personal choice. If you have a favourite reagent, or if you are familiar with a particular reaction (or if you have a strong aversion to a reaction/reagent) then this will affect your choice. Also you need to bear in mind the efficiency of the reactions involved, and any potential side reactions (for example, self-condensation of PhCOMe in method 4).
DEFINITIONS
TARGET MOLECULE (TM) what you need to make
RETROSYNTHETIC ANALYSIS the process of deconstructing the TM by breaking it into simpler molecules until you get to a recognisable SM
STARTING MATERIAL (SM) an available chemical that you can arrive at by retrosynthetic analysis and thus probably convert into the target molecule
DISCONNECTION taking apart a bond in the TM to see if it gives a pair of reagents
FUNCTIONAL GROUP changing a group in the TM into a different one INTERCONVERSION (FGI) to see if it gives an accessible intermediate
SYNTHON conceptual fragments that arise from disconnection
SYNTHETIC EQUIVALENT chemical that reacts as if it was a synthon
8 Some synthons and synthetic equivalents synthon equivalent(s)
R RCl, RBr, RI, ROMs, ROTs only when R = ALKYL
OH O
R R R R
OH O OH
Br R R , R
O O
R R
O O O O O
R R OEt , R Cl , R O R
R RMgBr, RLi, R2CuLi, other organometallic (alkyl; NOT "RH + base") reagents
O O O
CO2Et R R , R
nb// make sure you don't lose CH2 groups if you represent eg. RCH2 as R— (viz. make sure the product has the right number of carbon atoms!)
9 Latent Polarity
Think about some of the reactions we've looked at for carbonyl compounds:
O OH O A Nu Nu +
O O
: base B H O E
O +
E
O O Nu
C Nu O
E + + O
Nu
E i.e. O
etc. these polarities apply quite generally:
OH Br
+ + + + + +
NR NHR
+ + + + + +
10 The partial positive and negative charges indicate the latent polarity of the bonds in a molecule. They help us choose the synthons for key disconnections in a retrosynthetic analysis. viz.
OH OH
one of the disconnections we saw earlier.
Latent polarity in bifunctional compounds
Consider a 1,3-disubstituted molecule, e.g.
Latent Polarities: O OH
O OH starting from C=O Ph
Ph O OH starting from C Ph
When the latent polarities in a bifunctional molecule overlap they reinforce each other, this is termed CONSONANT POLARITY. In these circumstances the analysis is straightforward. thus,
O OH O OH O PhCHO + Ph Ph
O O OH i) base ii) PhCHO Ph
Similar principles apply for other 1,3-systems:
O O OH OH O NR2
etc.
11 The same applies to 1,5-disubstitution
O O O O
R R R R
O e.g. + R
O NaOH O O O R R R
R
But what about 1,4-disubstitution?
O O
O O
The polarities don't overlap and are termed DISSONANT. Any disconnection we try will result in a synthon that has the "wrong" polarity.
O O
synthons
O O
O ? equivalents
+ base
One way to get around this is by judicious placement of heteroatoms:
12 O O Br
O O base O
Br O
The German word UMPOLUNG, meaning polarity reversal is used to describe the situation where the polarity in a compound is deliberately changed to facilitate a particular reaction. example:
reacts with nucleophiles O
+ H
HS SH
cat. BF3·OEt2
ng S S lu acidic proton o H p (pKa ~ 32) um n-Butyllithium
reacts with S S electrophiles + Li
13 Equivalents for synthons with reversed polarity synthon equivalent(s)
OH O OH
Br R R R , or R
O
O O O R Br Br R R , or
O OEt
+ sec-BuLi Me
O S "Corey- + n-BuLi Seebach reaction" R R S
S S O + n-BuLi
S H , or MeNO2 + base ("Nef reaction")
O NaCN
HO footnote to table OEt OEt OEt s-BuLi E
E (VERY strong base) Li ethoxyvinyllithium EVL + H3O similarly from acetylene: O OH i) base E H O+ 3 tautom. E ii) E HgO E
14 Latent polarity and FGI (a quick consideration)
O
Ph Ph
FGI O Leads to O OH obvious disconnection O O Ph + Ph + Ph + + Ph Ph Ph OH + + {PhCOMe & PhCHO} Mismatched Matched (dissonant) (consonant)
SURVEY OF FUNCTIONAL GROUP INTERCONVERSIONS note: This is not supposed to be an exhaustive list of organic chemistry, nor is it supposed to tell you anything you don't already know [for more information see relevant lecture notes or consult a textbook]. The idea is to demonstrate how functional groups are related.
--note 2: the schemes are not repeated here; consult the paper copy that was given out during the lecture. You were there, right?
15 Strategy in retrosynthesis
1) Consider different possibilities. Try a number of disconnections and FGI's. Try to keep the number of steps down, and stick to known & reliable reactions. In real life, a synthesis has to be economically viable. 2) Whenever possible, go for a convergent route rather than a linear one, as this will lead to a higher overall yield
eg. ABCDEF ABCDE + F
ABCD + E
t ABCD + EF l i
n n
e e
g a
r r
e
v n ABC + D o
c AB + CD , E + F
AB + C A + B , C + D A + B
Linear vs. convergent synthesis: assume 80% yields (optimistic!)
Linear:
step 1 2 3 4 5 ...10 ...15 A AB ABC ABCD ABCDE ABCDEF A...K A...... P approx overall yield: 80% 64% 51% 40% 32% ...10% ...3.5%
Convergent:
A AB ABCD C CD ABCDEF A...K E EF G...K A...... P L...P 80% 64% 51% 40% 32%
The purely convergent synthesis is an ideal; virtually all real synthesis are linear to some degree
16 3) Aim for the greatest simplification
make disconnections towards the middle of the molecule (this is more convergent anyway) disconnect at branch points use symmetry where possible
eg. (towards the middle) O O O O
Ph Ph
O O O base O Ph MVK Ph methyl vinyl ketone MVK
eg. (at branches) O O
CO2Et CO2Et Ph Ph
O
O NaOEt CO2Et Ph CO2Et Ph Br
eg. (look for symmetry) O O O
HO HO
O O O
NaOEt HO H2O self-condensation
17 4) Add reactive functional groups at a late stage in the synthesis so they aren't carried through steps where they could react to give side products.
NMe NMe NMe
O DiBAlH m-CPBA
O OH OH
OH O DiBAlH R' R' R R
Alternatively, potentially reactive groups can be protected or masked so they don't react, eg. reduction of an ester in the presence of a ketone
O OH HO O O CO2Me CO2Me Ph cat. TsOH Ph Ketal (stable to LiAlH bases and 4 nucleophiles) Et2O
O + H3O O O
Ph OH Ph OH
Note that protection strategy requires two extra steps (must be efficient); better syntheses minimise the use of protecting groups.
A masked group is a functional group that is introduced and can be converted into a different one at a later stage ( remember EVL)
O OEt OEt OEt OEt sec-BuLi RX steps + H3O
Li R R' R'
masked acetyl group
18 5) Sometimes it helps the retrosynthesis if you add a functional group to facilitate bond formation (Functional Group Addition). An example of this is acetoacetic ester synthesis:
O O O OEt Thus:
O O O O FGA discon. discon. CO2Et CO Et Bu CO2Et 2
(acetoacetic ester is much more easily deprotonated than acetone)
The synthesis therefore is
O O O O O CO H + 2 CO2 NaOEt NaOEt CO2Et H3O Bu CO2Et Bu CO2Et Bu MeI BuBr
The strategy of FGA applies especially in the case of molecules containing no reactive functional groups:
OH FGA OH discon.
OH
Br H2 H O+ i) Mg/Et2O 3 T.M. Pd/C ii) O
alternatively:
19 FGA discon. T.M.
O O
Zn-Hg O (p-Tol)2CuLi T.M. O HCl
nb// 2 ArLi + CuCl Ar2CuLi + LiCl
20 Ring Closing Reactions Synthesis of carbocyclic molecules Same approach as to acyclic systems. The probability of reaction between two functional groups is higher if: a) reaction is intramolecular (faster reaction) b) the distance between the two groups is shorter e.g. Intramolecular alkylation:
EtO2C CO2Et EtO2C CO2Et EtO2C CO2Et
X
EtO C CO Et EtO2C CO2Et 2 2 NaOEt NaOEt EtO2C CO2Et Br BrCH2CH2CH2CH2Br
Intramolecular acylation eg. the Dieckmann cyclisation; especially good for 5-membered rings:
O O
CO2Et CO2Et
O NaO OEt CO Et CO2Et NaOEt 2
CO2Et EtO2C condensation:
O O O
OH OH
O O
O O t-BuOK
OH
21 Bicyclic molecules are prepared from cyclic precursors following similar principles.
OTs diethyl malonate
DEM CO2Et DEM 2 NaOEt
CO2Et CO2Et OTs EtO2C
O
CO2Et NaOEt
CO2Et
CO2Et
O O KOH O
A special example of condensation is the Robinson annulation (opinions vary as to the spelling). It has been widely used in classical steroid synthesis. It involves Michael addition followed by intramolecular cyclisation:
MVK? O see above O O t-BuOK
MVK
base
OH O O base
"signature" of Robinson annulation
22 Medium and Large Rings (8-11 membered and 12+)
Intramolecular reaction is less favoured with bigger rings. Often, high-dilution conditions and slow addition can be used to suppress intermolecular reaction and hence promote ring closure. eg.
O NaH MeO2C(CH2)7CO2Me (CH2)6 ester added over
nine days CO2Me similarly
O
" EtO2C(CH2)14CO2Et (CH2)13
CO2Et
Another reaction which works well for such systems is the acyloin reaction. This is the intramolecular dimerisation of a diester via a one-electron reduction. The reaction is heterogeneous, taking place at the surface of molten sodium metal, so high dilution is not required. eg
O Na, xylene, EtO2C(CH2)8CO2Et (CH2)8
OH and
O
" EtO2C(CH2)16CO2Et (CH2)16
OH
Cycloaddition reaction (Diels-Alder)
Generic reaction (in retrosyntheic terms):
X X X = EWG (CHO, CO2R, CN)
electron rich electron poor
23 eg
CO Me 2 CO2Me
concerted reaction &
CO2Et
CO2Et
CO2Et CO2Et
These reactions are concerted reactions, usually they are highly stereospecific. This is because the reactions are governed by Frontier Orbital Theory. The actual rules of frontier orbital theory don't interest us at the moment, all we need is a simple guideline we can remember:
Unsymmetrical Diels-Alder reactions:
R R R
R' R' + R'
Major product Minor product
R R' R R' R +
R'
Major product Minor product note that the 1,3-disubstituted product is the minor product in both cases specific example: CH CH 3 CH3 3
CO2Me CO2Me + FGA now CO2Me use D-A 61% only 3%
24 Disconnections & Functional Group Interconversion in Aromatic Systems
Some reactions used in aliphatic systems don't apply for aromatic systems (SN1 and SN2 reactions, for example, are extremely unfavourable for ArX. There is a whole bunch of other reactions that apply for aromatic systems. eg. O O
R R PhH + RCOCl + AlCL3 Friedel-Crafts acylation
O
RCOCl R AlCl3
NH 2 NO2 NO2 PhH + HNO3 + H2SO4 aromatic nitration
NO2 NH2 Sn/HCl fuming HNO3 or H2, Pd/C H2SO4
Br PhH + Br2 + FeBr3 Br aromatic bromination
Br
PhN2X + CuBr Sandmeyer reaction
NH Br 2 i) NaNO2/HCl Br2/FeBr3 ii) CuBr
(can get dibromination) (only monobromination)
25 Some other reactions
CO2H - KMnO4, (OH )
H2O - t-BuOH
CHO H2CO/POCl3/DMF Vilsmeier-Haak formylation
H2CO/HCl Cl chloromethylation ZnCl2
I R
R2CuLi
The last reaction above is a particularly useful application of organocopper reagents. Although the mechanism is quite complicated, it's the result we're interested in at the moment. It's a transformation that is not always easy to achieve by more conventional means.
In planning synthesis of polysubstituted aromatics, the order of reactions is important to ensure that the reagents are compatible and to take advantage of the directing effect of existing substituents:
Group Directs Activation
NH2, NR2 (more) - OH, O activating NHAc, OR ortho/para-* alkyl/aryl/vinyl CO - 2 neutral X (halogen) CO2H CN COR, CHO meta- deactivating SO3H CX3 NO2 (more)
* note that ortho/para- mixtures can be formed and may have to be separated
26 Examples
CO Et CO H 2 CO2H 2
H N O N 2 H2N 2 H2N
benzocaine (painkiller)
from toluene CO H CO2H 2
HNO 3 KMNO4 H2 Pd/C EtOH T.M. H2SO4 H+
NH NO2 2 NO2 OH Br OH Br Br Br
HO2C CO2H
I I NH2 I NH2 building block for homogeneous catalyst synthesis Br Br Br
NaOH i) Ac2O/AcOH i) NaNO2/HCl
ii) Br2 57% EtOH ii) KI
NH2 NHAc NH I Br 2
HO2C CO2H
KMnO BH3·SMe2 4 T.M. aq. t-BuOH
I nb// acetanilide prevents polybromination
Birch Reduction
Partial reduction of aromatic systems by (usually) sodium in liquid ammonia. It's an example of dissolving metal reduction. Such methods used to be quite popular but most applications have been replace by modern hydride reagents. Dissolving metal reduction does still have it's uses though, and the Birch reduction is one of them. (also recall the specific reduction of alkynes to trans-alkenes) The typical conditions involve liquid ammonia (bp. −33 °C) and sodium metal, in the presence of a proton source (usually an alcohol, EtOH).
27 EWG EWG Na, NH3 (l), EtOH
eg EWG = CO2H, NO2
EDG EDG "
eg EDG = Me, OMe
Examples
can be useful because... O O OMe OMe Na, NH3 (l), EtOH
OMe OMe OMe " nb// not
can you see why?
OMe OMe OMe " and not
OMe OMe OMe
CO2H CO2H "
28 Fusing Rings onto aromatic systems
The classical Hayworth naphthalene synthesis. The fused aromatic system is formed by dehydration of a tetralin intermediate, which is prepared from an existing benzene ring and succinic anhydride.
O O
discon. + O
CO2H FGI O
Thus:
O O O O
Zn-Hg/HCl
Clemmensen AlCl 3 HO2C HO2C
i) SOCl2 ii) AlCl3
Pd/C i) RMgx tetralone + ii) H3O
R R O 1-subtitution (aka -) via enamine RBr
Pd/C i) LiAlH4 ii) H O+ R R 3 R 2-subtitution (aka -) O other substitution patterns can be similarly obtained.
29 Blocking positions in aromatic rings
Functional groups that are introduced reversibly, or can be easily cleaved under mild condtions, can be used to access otherwise hard-to-make compounds
Et Et Et Et
Br Br SO3 Br2 dil.
H SO 2 4 FeBr3 H2SO4
SO3H SO3H
Br Br
NH2 NH2 i) NaNO /HCl Br2 2
ii) H3PO2 Br Br Br Br
Transformations of Aromatic Systems--(Summary Scheme)
Consult the handout.
30 Overall Summary
To devise a synthesis:
1) Examine the TM; recognise functional groups and key structural features. In an exam you may be given a SM, if this is the case, check how it relates to the TM 2) Use FG's present to help indicate disconnection points. Use latent polarities, umpolung and FGA to help if neccessary 3) Consider FGI's appropriate to the TM; consider disconnections at branch points and heteroatoms. Be convergent—disconnect between FG's separated by a couple of carbon atoms 4) Keep the number of steps as low as reasonably possible, but do use protecting groups where neccessary 5) Disconnect to good SM's:
straight chain monofunctional compounds branched monofunctional compounds containing six carbon atoms or fewer (for these purposes, including allyl, alkenyl and cycloalkyl compounds) simple mono- and disubstituted benzenes common bifunctional compounds (acetoacetate esters, malonate derivatives etc.) hint: concerning regents & SM's...have you seen them before (like in tutorials?)
Further reading
I take full responsibility for any mistakes and tyops, after all, I'm just a man. I encourage all students consult with higher authorities, and you could do a lot worse than look at some of these:
o Organic Synthesis: The Disconnection Approach, S. Warren o Classics in Total Synthesis I & II, K.C. Nicolaou et al. o Advanced Organic Chemistry, J. March o Comprehensive Organic Transformations, R.C. Larock o Protective Groups in Organic Synthesis, T.W. Greene and P.G.M. Wuts
Go to the Library, it's free to get in.
31