Polar Covalent Bonds
- No true dividing line exists between ionic and covalent bonds (better to rely on general observations). - The ionic character of a bond (how ionic it is) is also determined by ΔEN (eg. bond with ΔEN = 1.7 has 50 % ionic character)
- electrons in bonding pair spend more time closer to the more electronegative element
- atom with higher electronegativity (EN) will attract bonding electrons more strongly, this atom therefore has a partial negative charge(δ-), the less electronegative element has a partial positive charge (δ+)
- The slight difference in charge within a covalent molecule is called a dipole (two poles)
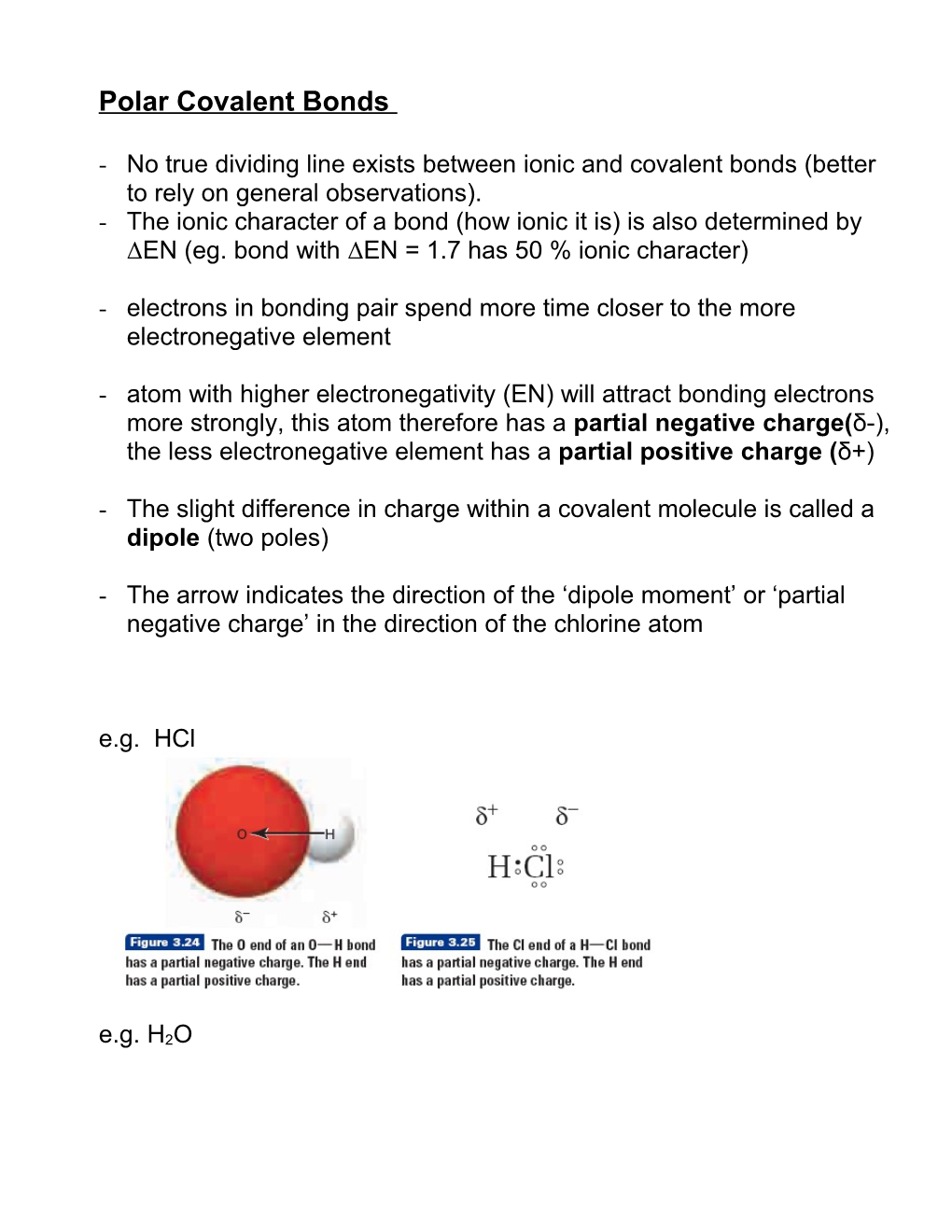
- The arrow indicates the direction of the ‘dipole moment’ or ‘partial negative charge’ in the direction of the chlorine atom
e.g. HCl
e.g. H2O - the negative end of the molecule tends to attract the positive end of a molecule, resulting in higher mp and bp for that molecule - molecules which have polar bonds can result in polar molecules, but not always it depends on the shape of the molecule
Molecular Shapes
- polar molecule results when a molecule contains polar bonds in an unsymmetrical arrangement. (NH3, H2O)
- a symmetrical molecule can contain polar bonds(the bond dipoles to cancel each other) e.g CO2, CCl4
- Compounds that are made up of non-polar molecules generally have lower melting points and boiling points than compounds that are made up of polar molecules. Guidelines for predicting polar and Non-polar Molecules (for your information)
Type Description Examples Polar AB - Diatomic compounds of different CO HAx elements HCl, HF AxOH - Molecules with a single H C2H5OH OxAy - Any molecule with an OH at one H2O NxAy end NF3, NH3 - Any molecule with an O at one end - Any molecule with an N at one end
Non Ax - All molecules of a single element Cl2, N2 polar - Many carbon compounds CxAy including organic solvents, fats and CO2, CH4 oils)