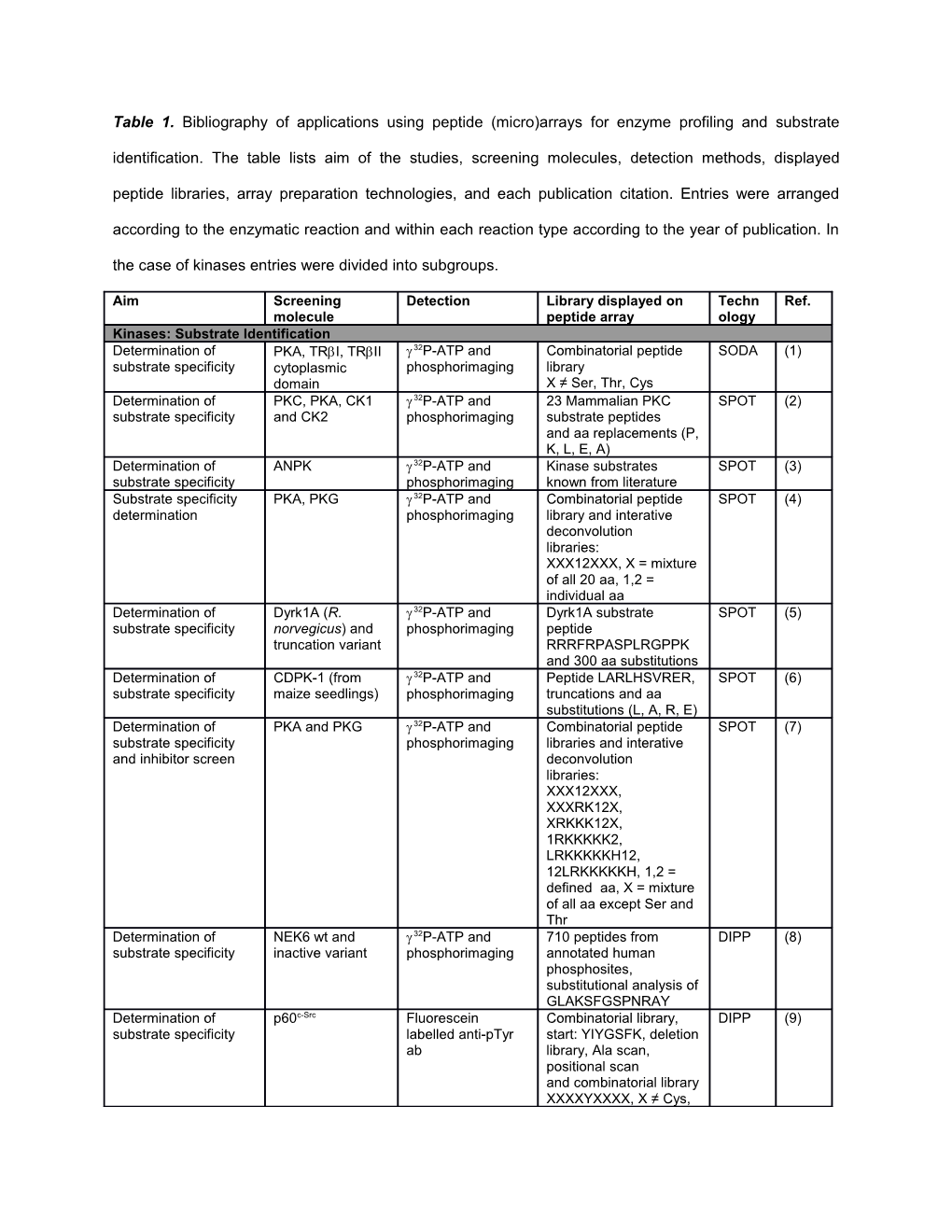
Table 1. Bibliography of applications using peptide (micro)arrays for enzyme profiling and substrate identification. The table lists aim of the studies, screening molecules, detection methods, displayed peptide libraries, array preparation technologies, and each publication citation. Entries were arranged according to the enzymatic reaction and within each reaction type according to the year of publication. In the case of kinases entries were divided into subgroups.
Aim Screening Detection Library displayed on Techn Ref. molecule peptide array ology Kinases: Substrate Identification Determination of PKA, TRI, TRII 32P-ATP and Combinatorial peptide SODA (1) substrate specificity cytoplasmic phosphorimaging library domain X ≠ Ser, Thr, Cys Determination of PKC, PKA, CK1 32P-ATP and 23 Mammalian PKC SPOT (2) substrate specificity and CK2 phosphorimaging substrate peptides and aa replacements (P, K, L, E, A) Determination of ANPK 32P-ATP and Kinase substrates SPOT (3) substrate specificity phosphorimaging known from literature Substrate specificity PKA, PKG 32P-ATP and Combinatorial peptide SPOT (4) determination phosphorimaging library and interative deconvolution libraries: XXX12XXX, X = mixture of all 20 aa, 1,2 = individual aa Determination of Dyrk1A (R. 32P-ATP and Dyrk1A substrate SPOT (5) substrate specificity norvegicus) and phosphorimaging peptide truncation variant RRRFRPASPLRGPPK and 300 aa substitutions Determination of CDPK-1 (from 32P-ATP and Peptide LARLHSVRER, SPOT (6) substrate specificity maize seedlings) phosphorimaging truncations and aa substitutions (L, A, R, E) Determination of PKA and PKG 32P-ATP and Combinatorial peptide SPOT (7) substrate specificity phosphorimaging libraries and interative and inhibitor screen deconvolution libraries: XXX12XXX, XXXRK12X, XRKKK12X, 1RKKKKK2, LRKKKKKH12, 12LRKKKKKH, 1,2 = defined aa, X = mixture of all aa except Ser and Thr Determination of NEK6 wt and 32P-ATP and 710 peptides from DIPP (8) substrate specificity inactive variant phosphorimaging annotated human phosphosites, substitutional analysis of GLAKSFGSPNRAY Determination of p60c-Src Fluorescein Combinatorial library, DIPP (9) substrate specificity labelled anti-pTyr start: YIYGSFK, deletion ab library, Ala scan, positional scan and combinatorial library XXXXYXXXX, X ≠ Cys, Tyr Profiling of phospho- CK2 32P-ATP and 2304 phosphorylated DIPP (10) specific antibodies and phosphorimaging and non phosphorylated detection of kinase or fluorescein peptides from annotated substrates labelled anti-pTyr human phosphosites for ab (Pt66, Sigma ab profiling and 11096 Aldrich), anti- pSer peptides covering the and pThr abs cytoplasmic domains of all human membran proteins and 700 biotinylated peptides for comparison of phosphorylation on microarrays and in solution Determination of Abl 32P-ATP and 1433 Random DIPP (11) substrate specificity for phosphorimaging sequences 13meric substrate prediction peptides with fixed tyrosine residue in the middle position Determination of M.musculus and 32P-ATP and 200 Peptides DIPP (12) substrate specificity H.sapiens TSSK3 phosphorimaging Determination of KPI-2 33P-ATP and 1152 Kinase substrate DIPP (13) substrate specificity phosphorimaging peptides of mixed species Substrate specificity Dyrk1A, Dyrk2, 33P-ATP and 720 Peptides derived DIPP (14) determination and Dyrk4 phosphorimaging from human comparison phosphosites Determination of MELK 32P-ATP and 192 Peptides comprising DIPP (15) substrate specificity phosphorimaging in vivo phosphorylation sites and peptides containing deletions or aa substitutions Determination of Dbf1-Mob1 (S. 32P-ATP and Oriented peptide library DIPP (16) substrate specificity cerevisiae) and phosphorimaging and interative inactive variant deconvolution libraries, N305A library: MAXXXXRXXSXXXXAK KK, proteome array containing 4400 GST proteins from S. cerevisiae and peptide microarray with 2296 annotated human phosphosites Kinase substrate Abl, Her2, Anti-pTyr ab (PY20, 10000 member PNA DCSD (17) screening VEGFR2/KDR Exalpha encoded peptide library: Biologicals), sec. F-Q-AA4-AA3-Y-AA2-AA1- ab Cy3 labelled Ile-Lys AA1, AA2, AA4: I, V, F, P, R, D-P, S, D-V AA3: I, V, A, P, R, K, -A, S, P Substrate specificity MPK1-3 (S. 33P-ATP and 976 Annotated kinase DIPP (18) determination lycopersicum) and phosphorimaging substrate consensus MPK6 (A. sites thaliana) Determination of Plk4/Sak (M. 32P-ATP and DYDIPTTENLYFQ, SPOT (19) substrate specificity musculus) wt and phosphorimaging substitutional analysis of inactive catalytic MSYYHHHHHH, domain substitutional scan of RKKKSFYFKKHHH and KSFYFKKHHH and 32 peptides from proteins containing putative phosphorylation sites and variants of positive peptides Determination of p60 c-Src, Abl and 32P-ATP and Array containing 144 Tyr SPOT (20) substrate specificity cytoplasmic phosphorimaging kinase substrate domains of EphA4 peptides, array containing all cytoplasmic Y ontaining motifs from 2- chimaerin, claudin-4 and FGFR1 and array containing systematic aa substitutions of Dok6 Y220 and RasGAP Y386 derived peptides and multiple substitutions Verification of the dual CK2, CK2 32P-ATP and CK2 derived peptides SPOT (21) specificity of CK2 phosphorimaging and sequences based on S. cerevisiae Frp3 togehter with positional scan Substrate specificity Protein kinase 7 33P-ATP and 1176 Kinase substrate DIPP (22) determination (P. falciparum) phosphorimaging peptides Use of peptide arrays c-Raf, MAP3K8 33P-ATP and 1176 Peptides DIPP (23) for prediction of kinase phosphorimaging containing human substrate specificities phosphorylation sites and second array containing 1024 peptides Substrate identification FLT3, inactive 32P-ATP and X-ray 384 Peptides derived DPCC (24) FLT3 K644A film imaging at –70 from known PDGFR and °C phosphorylation sites inhibitors STI571, AG1269 Substrate specificity p60c-Src, Abl, Phos-tag-biotin > 290 Tyr containing DIPP (34) determination EGFR, JNK1, followed by peptides, > 1100 Ser or Erk2, p38, PKA fluorescence Thr containing peptides labelled and new PKA streptavidin, Cy5 substrates: labelled anti-pTyr RKRRGSLDESD, ab RKRRASLDESD, RKRRGSFDESD, RKRRASFDESD, TKRSGSVYEPL, GAQAASPAKGE, ALRRApSLGW, ALRRASLGW, ALRRAALGW Analysis if PknB can PknB from S. 33P-ATP and 976 Disease related DIPP (26) phosphorylate human aureus phosphorimaging human kinase peptides and substrate phosphorylation sites specificity determination Determination of DAPK, Chk1 33P-ATP and 192 Kinase peptide DIPP (27) substrate specificity phosphorimaging substrates followed by p53 homology screen Substrate specificity PKA isoforms 33P-ATP and SDLRRTS, LRRTSIIGTI, SPOT (28) determination Tpk1 and Tpk2 phosphorimaging GRQRRLSSLSEF, from S. cerevisiae QQTRRGSEDDTY, LRRASLG and aa substitutions Kinases: Identification of phosphorylation sites in substrate proteins Determination of Lyn Anti-pTyr ab (4G10, 23 PKC derived SPOT (29) phosphorylation sites Upstate), HRP sec. peptides with central Tyr in PKC Ab residue Determination of PKC (S. scrofa) 32P-ATP and C-CAM-L derived SPOT (30) phosphorylation sites Edman sequencing peptides, Glu variants in large cell-cell and Ala screen adhesion molecule (C- CAM-L) Detection of Gsk-3and IL-1 32P-ATP and p65 peptide scan and SPOT (31) phosphorylation sites stimulated Hela phosphorimaging systematic mutagenesis on p65 lysate of Ser, Thr and Tyr to Ala Detection of PKA 32P-ATP and 20mer peptides SPOT (32) phoshorylation site in phosphorimaging encompassing C- AKAP-Lbc terminus of AKAP-Lbc Determination of CK2 CK2 32P-ATP and SSRP1 derived peptides SPOT (33) phosphorylation sites phosphorimaging containing Ser or Thr in SSRP1 and Ala variants Identification of PKA, Akt, PKC, 32P-ATP and 190 Peptides DIPP (34) kinases responsible for PKC, PKC, phosphorimaging encompassing 95 identified CK2, p38, Erk1, phosphosites from MS phosphorylation sites Cdk5 analysis and control peptides Phosphorylation site Recombinant 33P-ATP and Overlapping 13meric SPOT (35) determination human EphA4 phosphorimaging peptides corresponding receptor tyrosine to the juxtamembran kinase region of hEphA4 Phosphorylation site Recombinant 33P-ATP and Overlapping 13meric SPOT (35) determination human EphA4 phosphorimaging peptides corresponding receptor tyrosine to the juxtamembran kinase region of hEphA4 Identification of the PKA, Akt1, PKC, 32P-ATP and NEAIRTSTLPRNSGA, DIPP (36) kinase responsible for PKC, PKC, phosphorimaging NEAIRTSVLPRNSGA T840 phosphorylation PDK1, Cdk5, Raf- and peptides containing of GluR1 1, p38, MEK1, consensus Erk1, Erk2, Rsk2, phosphorylation sites JNK3, Gsk3, CaMK2, CK1, CK2, S6K, Rock2, TBK1, p60 c-Src, Fyn, Fes, Pyk2 Validation of new c-Src p60 c-Src 32P-ATP and 312 Peptides DIPP (37) substrates identified by phosphorimaging encompassing all Tyr mass spectrometry residues from 14 selected proteins and Phe variants Phosphorylation site CaMK2 32P-ATP and 23 Selected peptides SPOT (38) determination phosphorimaging covering Ets-2 Ser or T residues and positive control pp28 Validation of kinase Plk1, Aurora A 32P-ATP and 358 Identified spindle SPOT (39) phosphorylation sites phosphorimaging phosphorylation sites and VTELNDSRSECI and aa scan Phosphorylation site PKA 32P-ATP and 20mer peptides SPOT (40) determination phosphorimaging encompassing intracellular loop of rat cardiac Na+-Ca2+ exchanger NCX1, and substitution analysis of identified substrates Kinases: enzymatic activities in cell lysates and kinome profiling Comprehensive PKA, lysate of 33P-ATP and 192 Kinase peptide DIPP (41) description of LPS and phosphorimaging substrates phosphorylation unstimulated events induced by LPS PBMCs, stimulation of PBMCs MAPK and inhibitors PD98059, SB203580 Analysis of kinase Lysates from M. 33P-ATP and 1152 Kinase substrate DIPP (42) activities in cell lysate musculus MAFs phosphorimaging peptides of mixed and M. musculus species MLCECs ± BMK1 knockout Fingerprinting effects Lysate of anti-CD3 33P-ATP and 1176 Peptides DIPP (43) of DEX on T- and anti-CD28 ab phosphorimaging containing kinase lymphocyte kinome stimulated CD4+ substrate consensus cells treated ± sequences DEX Comparison of kinome Tissue lysates 33P-ATP and 1176 Kinase specific DIPP (44) profiles of Barrett’s phosphorimaging consensus sequences esophagus, normal squamous esophagus and normal gastric cardia Investigation of the Lysate of insulin 33P-ATP and 1176 Peptides DIPP (45) effect of DEX on stimulated 3T3 phosphorimaging containing kinase adipocyte and T- adipocytes treated substrate consensus lymphocyte kinome ± DEX and lysate sequences of anti-CD3 and anti-CD28 ab stimulated CD4+ cells treated ± DEX Detection of kinase PKA, PKC, MCF-7 Biotinylated Phos- LRRAXLW, YIXGSFK, DIPP (46) activity in cell lysate lysate incubated ± tag followed by LRVQNXLRRRR, X = S, Forskolin and H89 DyLight 647 or Cy3 pS, T, pT, Y, F, A labelled anti- streptavidin ab Analysis of kinase Lysates from 2 33P-ATP and 192 Kinase peptide DIPP (47) activities in lysates and 5 weeks old phosphorimaging substrates A. thaliana and from P. syringae infected A. thaliana Screening of renal Cortical tissue 33P-ATP and > 1100 Mammalian DIPP (48) kinase activities to lysates from phosphorimaging kinase substrate identify kinases sprague dawley sequences involved in angiotensin rats and Ren 2 II dependent rats treated ± hypertensive renal ACEi damage Tyrosin kinase profiling Zebrafish embryo Fluorescein 144 Tyr kinase substrate DIPP (49) in zebrafish embryos lysate (1 day post labelled anti-pTyr peptides fertilisation) and ab (PY20, Exalpha knockdown of Src Biologicals) family kinases, Fyn, Yes and Wnt11, Fyn and Yes kinase Tyrosine kinase Zebrafish embryo Fluorescein 144 Tyr kinase substrate DIPP (50) profiling in zebrafish lysate 3 and 5 labelled anti-pTyr peptides embryos days post ab (PY20, Exalpha fertilisation Biologicals) Determination of Lysates from P. 33P-ATP and 1152 Kinase substrate DIPP (51) kinase activities in cell pastoris, T. phosphorimaging peptides of mixed lysates to analyse aestivum, C. species phylogenetic relations albicans, A. of microarray thaliana, F. solani, phosphorylation D. melanogaster, patterns H. sapiens, M. musculus, D. discoideum and murine M Effects of Pichinde Cytoplasmic 32P-ATP and 1176 Peptides DIPP (52) virus infection on macrophages phosphorimaging containing human in signalling pathways in extract after mild vivo phosphorylation C. porcellus and lethal virus sites macrophages infection (P2, P18) 1d and 6d post infection Phosphorylation Cytosolic and 32P-ATP and 47 Peptides contaning DIPP (53) profiling of A. thaliana nuclear extracts, phosphorimaging known plant proteins cytosolic extracts phosphorylation sites after stress, SRPK4 (A. thaliana), MPK3 (A. thaliana) Detection of kinase p60c-Src, PKA, pTyr detection: 26 Peptides containing DIPP (54) activities in cell lysates EGFR, anti-pTyr ab (Pt66, kinase consensus motifs using SPR MAPKAPK-2, Sigma Aldrich) CaMK2, Akt, followed by SPR p38, JNK1, pSer/pThr PKC, Erk2, detection: Phos- PKC, Abl, InsR, tag-biotin followed Jak1, PKC and by streptavidin, inhibitors SU6656, anti-streptavidin ab H-89 and A431 and SPR cell lysate ± NGF stimulation and SU6656 addition Kinase profiling of SARS coronavirus 33P-ATP and 1152 Kinase substrate DIPP (55) virion components lysate phosphorimaging peptides of mixed species Fingerprinting of Lysates of FITC labelled anti- 144 Tyr kinase substrate DIPP (56) tyrosine kinase NUP214-Abl and pTyr ab (PY20, peptides activities BCR-Abl Exaplha expressing Ba/F3 Biologicals) cells incubated ± imatinib Fingerprinting of Lysates of human FITC labelled anti- 144 Tyr kinase substrate DIPP (57) tyrosine kinase dermal pTyr ab (PY20) peptides activities microvascular endothelial cells and of hemangioma endothelia cells Analysis of the effect Lysates from 33P-ATP and 1176 Kinase substrate DIPP (58) of aspirin and colon cancer cell phosphorimaging peptides celecoxib on cancer lines DLD1 and kinome HT29 treated with aspirin or with celecoxib Fingerprinting of Lysates of FITC labelled anti- 144 Tyr kinase substrate DIPP (59) tyrosine kinase androgen- pTyr ab (PY20, peptides activities sensitive prostate Exaplha carcinoma cell line Biologicals) LNCaP Analysis of the PKA, CaMK2, 32P-ATP and 600 Peptides DIPP (60) postsynaptic Erk2, Rock2, phosphorimaging corresponding to 300 M. phosphoproteome Gsk3, Fes, musculus in vivo JNK3, Akt1, phosphorylation sites in PKC, PKC, 92 synaptic proteins PKC, p70S6, together with A, V or F Rsk2, Erk1, Cdk5, variants of the S, T and p38, CK1, CK2, Y residues and c-Raf, MEK1, additional peptides PDK1, TBK1, Fyn, containing kinase Pyk2 consensus motifs and a priming array containing 200 peptides from multiple phosphorylated sequences togehter with A, V or F variants of the S, T and Y residues and pS, pT and pY substitutions Kinome profiling for Chondrosarcoma 33P-ATP and 1024 Kinase substrate DIPP (61) putative target lysates from 9 phosphorimaging peptides detection and analysis primary cell lines of differences in and OUMS27, primary and metastatic CH2879, SW1353 UM lysates and C3842 cell lines and lysates from 2 MSC cultures and 5 colorectal carcinoma cell lines and inhibitors PP1, PP2 and PP3 and Mel270 cell lysate treated ± p60 c-Src siRNA Investigation of the Lysates from A. 32P-ATP and 1176 and 1024 Kinase DIPP (62) role of phosphorylation thaliana treated phosphorimaging substrate peptides in plant sugar with water, response sorbitol, glucose or sucrose Tyrosine kinome Uveal melanoma FITC labelled anti- 144 Tyr kinase substrate DIPP (63) profiling (UM) cell lysates: pTyr (PY20, peptides 8 primary UM, 3 Exalpha UM metastases, 3 Biologicals) fresh UM samples and 3 liver metastases Kinome profiling Lysates from A. 32P-ATP and 960 Kinase consensus DIPP (64) thaliana fed phosphorimaging sequences selected for sucrose or sorbitol their importance in mammalian signal transduction and 1152 kinase substrate peptides of mixed species Detection of kinase p60 c-Src, cytoslic Cy5 anti-pTyr ab 840 Peptides DIPP (65) activities in cell lysates and membrane fraction of MCF-7 lysates, inhibitors PP2 and SU6656,, NGF, CHO and A431 lysates and lysates from M. musculus brain and skin Kinome profiling for the 29 pediatric brain Fluorescein 144 Tyr kinase substrate DIPP (66) detection of aberrant tissue lysates and labelled anti-pTyr peptides kinase activities in lysates from ab (PY20, Exalpha neoplastic tissues Wilms’ tumors, Biologicals) colon carcinomas, normal kidney and normal colon Kinome analysis of Lysates of 32P-ATP and 298 Peptides containing DIPP (67) monocytes monocytes from phosphorimaging proposed B. taurus B. taurus treated ± phosphorylation sites LPS togehter with 2 negative and 7 positive controls Fingerprinting tyrosine Lysates of FITC labelled anti- 144 Tyr kinase substrate DIPP (68) kinase avtivities leukemia samples pTyr ab peptides Kinome profiling and B-cell lysates from 33P-ATP and 1024 Kinase substrate DIPP (69) biomarker identification 88 Systemic phosphorimaging peptides Lupus Erythematosus patents and from 72 healthy controls Kinome profiling of MC3T3-E1 cell 33P-ATP and 1024 Kinase substrate DIPP (70) osteoblast adhesion lysate 2h after phosphorimaging peptides adhesion substrate subjection and control Kinome profiling of Lysates of two 33P-ATP and 1024 Kinase substrate DIPP (71) myxoid liposarcoma myxoid phosphorimaging peptides liposarcoma cell lines and primary cultures of four myxoid liposarcomas Screening of kinase Lysates of A. 33P-ATP and 1178 Kinase substrate DIPP (72) activities in response thaliana incubated phosphorimaging peptides of mixed to Jasmonate and with jasmonic acid species salicylate treatment and/or salicylic acid Tumor kinase profiling Biopsy lysates Fluorescently- 144 Tyr kinase substrate DIPP (73) to predict from rectal cancer labelled Anti-pTyr peptides chemotherapy patients ± sunitinib ab response Fingerprinting tyrosine Lysates from 4 Fluorescently- 144 Tyr kinase substrate DIPP (74) kinase avtivities different cell lines labelled Anti-pTyr peptides and their matching ab phosphatase and tensin homolog (PTEN) knockdown sublines Fingerprinting tyrosine Lysates from Fluorescently- 144 Tyr kinase substrate DIPP (75) kinase avtivities paediatric brain labelled Anti-pTyr peptides tumor tissue ab Kinases: inhibitor identification and characterization Identification of Enzyme I of the 32P- Combinatorial peptide SPOT (76) peptides that inhibit bacterial phosphoenolpyruva library and interative enzyme I of the phosphotransferas te and deconvolution bacterial e system phosphorimaging libraries: phosphotransferase XXXXXB1HB2XXXXX, system XXXXB1HB2XXXX, XXXXXB1CB2XXXXX B = defined, X = randomized Identification of c-GMP dependent 32P- Combinatorial peptide SPOT (77) peptidic inhibitors kinase autophosphorylated library and interative PKG and deconvolution phosphorimaging libraries: XXX12XXX, XXXRK12X, XRKKK12X, 1RKKKKKH12, 12LRKKKKKH 1,2 = defined aa, X = mixture of all aa except Ser, Thr and Ala screening of selected peptides c-Src 33 Determination of Ki p60 and P-ATP and YGEFKKK, FGEFKKK, DIPP (78) values for inhibitors inhibitors surface plasmon pYGEFKKK, YAAPKKK, quercetin, resonance (anti- LRRASLG tyrphostin, A47 pTyr ab, BD and PP1 biosciences) Investigation of HUVEC lysate 33P-ATP and 1152 Kinase substrate DIPP (79) signalling pathways treated ± phosphorimaging peptides of mixed influenced by spongistatin 1, species spongistatin 1 combretastatin A4 phosphate or vinblastine Prediction of kinase HT29 lysate FITC labelled anti- 144 Tyr kinase substrate DIPP (80) inhibitor resistance in transfected with pTyr ab (PY20, peptides tumors – profiling of p60 c-Src construct Exaplha repsonse to MTKI of or empty vector Biologicals) 27 cell lysates to incubated ± PP2 identify signature or JNJ-26483327 peptides (=MTKI), HCT116 and SKOV-3 lysate with p60 c-Src siRNA, KatoII,SNU-5, MKN45, NSCLC.H441, NCI-N87, SNU484, NSCLC- H322 and Caco2 lysates incubated ± JNJ-38877605, EGF stimulated lysates and lysates from 8 xenograft tumors incubated ± MTKI Analysis of a putative Mesenchymal 33P-ATP and 1152 Kinase substrate DIPP (81) kinase inhibitor stem cell lysates phosphorimaging peptides of mixed incubated ± species, 12 control inhibitor peptides PTK787/ZK22258 4 Analysis of the effect A549 cell lysates HRP conjugate 384 Peptides derived DPCC (82) of Pazopanib and treated ± anti-pTyr ab (R&D from known Lapatinib on A549 Pazopanib or Systems) phosphorylation sites kinome Lapatinib or both Effects of anti- PBMC lysates 33P-ATP and 1176 Mammalian in vivo DIPP (83) CD45RB ab on CD45 after incubation phosphorimaging kinase consensus activity with anti-CD45RB peptides ab Hydrogel based Lysates from NCI- Anti-pTyr ab (4610 AEEEEYFELVAKKK Diff (84) microarray for H23, NCI-H1650, Upstate), sec. ab assessment of EGFR PC9, H1650-ER, HRP labelled activity and sensitivity PC9-GR and to inhibitors in cell K562 lysates and lysates of drug inhibitors erlotinib, resistant tumors gefitinib, CL- 387,785, BIBW- 2992, CI-1033, PKI-166 Identification of HUVEC lysate 33P-ATP and 1152 Kinase substrate DIPP (85) signalling pathways treated with TNF- phosphorimaging peptides of mixed influenced by ± kinase species flavopiridol inhibitor flavopiridol Kinases: assay development/optimization Method for substrate PKA, PKG 32P-ATP and Combinatorial peptide SPOT (86) specificity phosphorimaging library and iterative determination using deconvolution: peptide libraries XXX12XXX X = mixture of all 20 aa, 1,2 = individual aa and peptides with different length and Ala or D-Ala variants as putative inhibitors Kinase assay on PKA, PKC, CK1, 32P-ATP and Kinase substrate SPOT (87) cellulose membranes CK2 phosphorimaging peptides: RRASVA, QKRPSQRAKYL, DDDDEESITRR, DDDSDDDAAA and Ser to Ala variants, PKA substrates with altering length: AAAARASVA, AARRASVA, RRASVA, RRASVAAA, RRASVAAAAA, RRASCAAAAAA and 9 PKA substrate peptides: LRRASVA, LRRASLG, RRASVA, LRKASLG, LHRASLG, LRHASLG, LKRASLG, LARASLG, LRAASLG Method for substrate PKA 32P-ATP and Combinatorial peptide SPOT (88) specificity phosphorimaging library and interative determination using deconvolution peptide libraries libraries: XXX12XXX, X = mixture of all 20 aa, 1,2 = individual aa Demonstration of p42 MAPK, PKA, 33P-ATP and Kemptide, Elk1, protein DIPP (89) applications for protein CK2 phosphorimaging kinase inhibitor 2 and peptide microarrays Demonstration of p60c-Src 33P-ATP and EEIYGEFF DIPP (90) versatility of peptide phosphorimaging arrays for functional assays Development of an p60c-Src, PKA FITC-labelled YIYGSFK, ALRRASLG, DIPP (91) antibody based phospho-specific YIYGpSFK, method to detect abs ALRRApSLG kinase activity Method for detection of Erk2 Anti-pElk1 (S383, KGRKPRDLELP, SPOT (92) weakly interacting NEB) ab, HRP FWSTLSPIAPR, protein-protein motifs labelled sec. Ab MNGGAANGRIL using paired peptides Validation of phospho- Abl ATP or -thio-ATP. Kemptide, Abl tyrosine CLPP (93) specific dye on solid Phosphorylation kinase peptide and supports was detected with Ca2+/calmodulin kinase Pro-Q Diamond substrate (glycogen dye or synthase 1-10) in 15 thiophosphorylation different dilutions on was detected with polyacrylamide coated thiolreactive slides BODIPY 630/650 methyl bromide reagent Method for Abl, PKA ProQ Diamond Abl peptide, CaMK2 DIPP (94) fluorescence detection (Invitrogen) peptide, Kemptide of phosphorylation on peptide microarrays Different methods on PKA, CK2, Gsk3, 32P-ATP and 3 Arrays containing DIPP (95) peptide arrays for PDK1, Tie2 phosphorimaging either 710 human kinase research phosphorylation sites or 2234 peptides ± priming phosphorylations or 1394 kinase activation loop derived peptides and peptides covering the cytoplasmic domain of Tie2 as overlapping peptide scan Method to evaluate p60c-Src, PKA, MALDI-TOF/MS IYGEFKKKC, DIPP (96) kinase activities on PKG, CaMK2, LRRASLGC, peptide microarrays CKI, Abl, Erk RKRSRAEC, using MALDI-TOF/MS inhibitors KRQQSFDLFGC, CKRRALpSVASLPGL, IYAAPKKKL, TGPLSPGPFGC Assay to detect PKA and inhibitor Biotin-ATP followed LRRASLG, LRRAGLG DIPP (97) phosphorylation using H89 by avidin stabilized gold nanoparticle gold particles, silver probes enhancement and resonance light scattering Development of a Abl, p60 c-Src, CK1, MALDI-TOF/MS AIYAAPFKKGC, DIPP (98) microarray based multi Erk1, PKA, PKC, EEIYGEFE, AKKKC, analyte assay which PKG and CKRRALpSVASLPGL, allows incubation with inhibitors Gleevec, TGPLSPGPFGC, different enzymes in Gö6850 and LRRASLGC, one experiment lysate from K562 AKIQASFRGHMARKKG cells that C, RKRSRAEC, overexpress Bcr- AIpYAAPFKKGC, Abl ± Gleevec, LRRApSLGC peroxide vanadate and calynuclin A treatment Method for detection of PKA 32P-ATP or CGGLRRASLG, Ala DIPP (99) phosphorylation on biotinylated Zn2+ substitution and Ser peptide microarrays chelate compound phosphorylated, followed by CGIYGEFKKK and Tyr streptavidin and phosphorylated SPR, anti-pSer (PSR-45, Sigma Aldrich) or anti-pTyr (PT-66, Sigma Aldrich) ab followed by SPR Kinase inhibition assay PKA and inhibitors ProQ Diamond Kemptide Diff (100) using sol-gel derived H7, H89 (Invitrogen) multicomponent microarray c-Src Method for screening p60 , B-Raf Anti-pTyr ab Poly(E4-Y)10 peptide DIPP & (101) of kinase – chemical (V599E), KDR, (pTyr100, Cell SPNS interaction using Met, Flt3 (D835Y), signalling), sec. aerosol deposition of Lyn, EGFR, Alexa 555 ab nanodroplets PDGFR, Tie2 containing the and inhibitors biologically active staurosporine, compound PP2, GW5074, KDRI and PDGRF 1-1 Microfluidic peptide PKA ProQ Diamond R(R/K)XSLG, X = any LDPS (102) microarray applications (Invitrogen) aa and pY, A and F variants of S Method for employing PKA and Biotin-ATP, LRRASLG, LRRAGLG DIPP (103) kinase inhibition based PKA inhibitors detection using on labelling H89, HA1077, avidin stabilized phopshorylation KN62, mallotoxin gold nanoparticles events using nanogold followed by silver particles enhancement and resonance light scattering Solution phase kinase CK1, Plk3, Akt1, Fluoresceinylglycin 900 Peptide substrates DCSD (104) assay SGK1, PKC, e amide followed encoded by an PKCz, PKA, by anti-fluorescein oligonucleotide Aurora A, Aurora ab (Roche), sequence composed of B, Chk2, CaMK2, biotinylated sec. ab annotated kinase MK2, Chk1, Cdk2, and strepatavidin- substrate sequences Cdk5, p38, CK2, Alexa555 or anti- and random sequences Abl, Lck, p60c-Src, pTyr-biotin ab EphB2, VEGFR2, (Invitrogen) Kit, Flt3, Jak2, followed by EGFR and streptavidin inhibitors PKI, phycoerythrin SrcI, TBB, staurosporine Fluorescence based PKA, Akt1 and -elimination RPRAASF, LRRASLG SPOT (105) assay for inhibitor followed by Michael phosphorylation staurosporine addition of thiol detection containing tetramethylrhodami ne Protocol for Different human 33P-ATP and 3280 Peptides covering DIPP (106) phosphorylation site kinases phosphorimaging all human peptidyl- determination or ProQ Diamond prolyl-cis/trans- followed by isomerases and 17181 fluorescence peptides covering the reading CMV proteome Protocol for kinome Plant tissues 33P-ATP and 1176 Kinase substrate DIPP (107) profiling using plant lysates, biopsie phosphorimaging peptides from different tissues, biopsies, cells lysates, cells organisms and a second and purified kinases lysate and purified arrays contaning 1024 in kinases vivo phosphorylation sites Kinetic studies of PKA PKA and inhibitors Anti-phospho ab, 140 Peptides derived DIPP (108) catalyzed staurosporine, sec. fluorescently from human phosphorylation in real AMP-PNP and labelled ab allowing phosphorylation sites time on a peptide array PKA inhibitor real time detection together with 4 peptide of fluorescence phosphopeptides and using a CCD detailed phosphorylation camera analysis of the peptide EILSRRPSYRKIL and kinetic analysis of CREB peptide and Kemptide (LRRASLG) Assay to identify PKC, Anti-pSer, anti-pThr 144 Ser/Thr Kinase DIPP (109) bisubstrate inhibitors ab (Cell signalling substrate peptides #2351, 9611, 9391), sec. ab FITC labelled Kinase assay using PKA and inhibitors Biotinylated anti- CGGALRRASLG, DIPP (110) Raman spectroscopy H89, HA1077, pSer ab (Sigma CGGALRRAGLG and gold nanoparticle KN62 Aldrich) followed by probes gold nanoparticles and silver enhancement Assay for the PKC and Anti-pSer/pThr ab, 30- and 10-meric Diff (111) development of a inhibitors TMP sec. ab Cy5 Peptides as MBP fusion depigmenting assay and labelled protein derived from bisindoylmaleimid tyrosinase e Protocol for A431 lysates ± Phos-tag-biotin Array containing 804 DIPP (112) measurement of SU6656 followed by Dylight peptides and second kinase activities in cell incubation, HepG2 649 conjugated array containing lysates lysates and streptavidin or Cy5 peptides EEIpYGEFD, lysates from M. labelled anti-pTyr EEIYGEFD, EEIFGEFD musculus tumors ab (Amersham) after A431 cell inoculation Protocol for HEK293 lysate ProQ Diamond 4198 Peptides DIPP (113) determination of (Invitrogen) representing overlapping kinase activities in cell peptide scans covering lysates all human peptidyl- prolyl-cis/trans- isomerases Kinases: peptide immobilization and array fabrication optimization Development of p60c-Src, PKA FITC-labelled YIYGSFK, ALRRASLG, DIPP (114) approaches for site phospho-specific KGTGYIKTG, specific immobilisation abs YIYGpSFK, of peptides ALRRApSLG, KGTGpYIKTG Review: peptide array c-Abl 32P-ATP or 720 peptides from DIPP (115) applications fluorescence- annotated human labelled anti-pTyr phosphosites ab (PY20) Immobilization PKA, p60c-Src FITC-anti-pTyr and YIYGSFK, ALRRASLG DIPP (116) strategies for peptides FITC anti-pSer ab on peptide microarrays (Sigma Aldrich) Development of an PKA, Cdc15 (S. 33P-ATP, anti-HRP Binding libraries: SPOT (117) oriented peptide library cerevisiae), ab sec. Ab AXXXXpS/pTXXXXA, approach MPM2, 3F3/2 and AXXXXpYXXXXA, PY20 ab AXXXXXpS/pTQDPFXX A, X ≠ Cys Kinase library: AXXXXXS/TXXXXA, X ≠ Ser, Thr, Cys Strategy for making Abl, PKA Cy3/Cy5 labelled Leptin-Kemptide, malic DIPP (118) peptide arrays using anti-pSer and pTyr enzyme-Kemptide, protein-peptide fusions ab (Sigma Aldrich) Leptin-Abl peptide, malic enzyme-Abl peptide Immobilization using p60c-Src,PKA and Anti-p-GFAP ab IYGEFKKK, IAGEFKKK, DIPP (119) poly(dT)-modified inhibitor AG213 (YC10 Medical and RRRVTSAARRS, peptides Biochemical RRRVTAAARRS Laboratories) and anti-pTyr ab (PY20, Exalpha Biologicals) Assay for p60 c-Src, PKA and Biotin-ATP followed Raytide, Kemptide DIPP (120) phosphorylation site inhibitors ellagic by streptavidin detection using gold acid, PP2, PP3, coated gold nanoparticle based genistein, nanoparticles and electrochemical herbimycin A electrochemical detection detection Optimized surface p60c-Src, PKA 33P-ATP or SPR CGIYGEFKKK, DIPP (121) chemistry for peptide imaging using Zn2+ CGGLRRASLG and immobilisation for chelate compound modifications phosphorylation analysis using different linker moieties Microarray assembly Pim1 and inhibitor 32P-ATP and KKRNRTLTK SPNS (122) by accoustic hb1277 phosphorimaging dispensing Phosphatases Regeneration of Potato acid 32P-ATP and Kinase substrate SPOT (87) phosphorylated SPOT phosphatase phosphorimaging peptides: RRASVA, membranes QKRPSQRAKYL, DDDDEESITRR, DDDSDDDAAA and S to A variants, PKA substrates with altering length: AAAARASVA, AARRASVA, RRASVA, RRASVAAA, RRASVAAAAA, RRASCAAAAAA and 9 PKA substrate peptides: LRRASVA, LRRASLG, RRASVA, LRKASLG, LHRASLG, LRHASLG, LKRASLG, LARASLG, LRAASLG PTP substrate PTP1B, PTP, Anti-pTyr ab AABX1pYX2BAAA, B = SPOT (123) specificity mixture (4G10, mixture of all 20 aa, X1, determination upstate, PY20 and X2 = individual aa, Tie2 PY69, Transduction peptides Laboratories, pTyr- VFSKYpYTPVLA, 100, Biolabs), HRP VFSKYpYpTPVLA, sec. Ab STAT5 peptide MAIESLNYSVYTTNS and pY, pS, pT variants and IR autophosphorylation sequence with all combinations of pY Substrate identification GST-fusion of Radioisotopic 7 Peptides derived from SPOT (124) substrate-trapping labelling of GST- cytoplasmic region of mutants of PTP- fusion proteins by GHR in non- H1, TCPTP, SAP- PKA mediated phosphorylated and 1, and PTP-1B phosphorylation tyrosine phosphorylated followed by form phosphorimaging Method for detecting PTP1B Anti-pTyr ab IR autophosphorylation SPOT (92) weakly interacting (Upstate), HRP site IYETDYpYRKGG protein-protein motifs sec. Ab and overlapping using paired peptides peptides covering the cytoplasmic sequence of IR Protocol for SPOT PTP1B Anti-pTyr ab IR peptide SPOT (125) applications for PTPs mixture (4G10, MTRDIYETDYYRKGG upstate, PY20 and and pY substitutions and PY69, Transduction SPOT double synthesis Laboratories, pTyr- covering the cytoplasmic 100, Biolabs), HRP IR domain as sec. Ab overlapping peptide scan togehter with the IR autophosphorylation peptide IYETDpYRKGG Phosphatase profiling K562 cell lysates MALDI-TOF/MS AIYAAPFKKGC, DIPP (98) using cell lysates treated ± Gleevec, EEIYGEFEAKKKC, peroxide CKRRALpSVASLPGL, vanadate, AIpYAAPFKKGC, calynulin A LRRApSLGC Construction of pTyr PTP1B, PTPµ Fluorescence 48 pTyr-containing DIPP (126) microarrays for imaging peptides phosphatase subsequent to specificity treatment with Cy5 determination labelled anti-pTyr ab Assay for substrate Lambda ProQ Diamond 87 phosphopeptides DIPP (127) specificity Phosphatase, AP, (Invitrogen) derived from know determination PP1, PP2B, PP2A phosphorylation sites ± Pin1 Peptide immobilization PTP1B Anti-biotin-strep- PDVLEYpYKNEHAK, DIPP (128) by click sulfonamide Cy5 RDINSLpYEVSRMY reaction Effects of anti- PBMC lysates 33P-ATP and 1176 Mammalian in vivo DIPP (83) CD45RB ab on CD45 after incubation phosphorimaging kinase consensus activity with anti-CD45RB peptides ab Identification of GST-fusion of Fluorescence 6057 pTyr-containing DIPP (129) physiological subtrates substrate-trapping imaging peptides derived from mutant of human subsequent to human phosphorylation Density-Enhanced incubation with Cy5 sites and 343 controls Phosphatase labelled anti-GST (CD148) ab Substrate specificity Cy3 or Cy5 Fluorescence 144 Putative Tyr DIPP (130) determination, labelled substrate- imaging phosphatase peptide identification of specific trapping mutants substrates substrates by dual- of PTP-1B, color readout TCPTP, SHP-1, SHP-2, and LMWPTP Substrate specificity PTP1B, TCPTP, ProQ Diamond 144 Putative Tyr DIPP (131) determination SHP1, SHP2, (Invitrogen) phosphatase peptide SHP1 and SHP2 substrates catalytic domain, LMWPTP Identification of GST-fusion of Fluorescence 6057 pTyr-containing DIPP (132) physiological subtrates substrate-trapping imaging peptides derived from mutant of human subsequent to human phosphorylation PTP-1B incubation with Cy5 sites and 343 controls labelled anti-GST ab Lysin Methyl-Transferases (PKMTs) Determination of the G9a 3H-AdoMet and Substitutional analysis of SPOT (133) contribution of aa for tritiumimaging a peptide covering the substrate recognition first 21 aa of H3 (all 20 aa and modified aa) and 45 peptides from putative new substrate proteins Substrate specificity Dim-5 (N. crassa) 3H-AdoMet and Overlapping peptide SPOT (134) determination tritiumimaging scan of the first 21 aa of H3 and K9A variant and substitutional analysis Substrate specificity Set7/9 3H-AdoMet and - 420 peptides SPOT (135) determination and tritiumimaging representing & identification of novel substitutional analysis of DPCC in vivo substrates histone H3 tail 1-21 - 133 predicted peptides derived from 118 different proteins - 216 peptides derived from histone H3 featuring 18 different posttranslational modifications Peptidyl-Prolyl-cis/trans-Isomerases Identification of Human Pin1 Sequential electro- Peptide scan of human SPOT (136) substrates blotting to PVDF- Cdc25C including membranes, phosphorylated S/T-P incubation of moieties PVDV-membranes with anti-Pin1 ab followed by secondary ab Determination of Trigger factor (TF) Sequential electro- Peptide scans (13meric SPOT (137) substrate specificity (E. coli) and TF- blotting to PVDF- peptides overlapping by fragments membranes, 10 residues) of E. coli incubation of proteins: elongation PVDV-membranes factor Tu, methionine with anti-TF ab biosynthesis enzyme, followed by isocitrate fluorescence dehydrogenase, labelled secondary glutamine-tRNA- ab synthetase, alkaline phosphatase, - galactosidase, galactose binding protein), L2 (a ribosomal protein), lambda cI, pro-outer membrane protein A, sigma 32, SecA, dehydrofolate reductase and yeast cytochrome B2, F1- and Su9- ATPase subunits, Photinus pyralis luciferase, RNaseT1 from Aspergillus oryzae and human PrP (prion protein) Determination of Trigger factor and Sequential electro- Peptide scans (13meric SPOT (138) substrate specificity DnaK (E. coli) blotting to PVDF- peptides overlapping by membranes, 10 residues) of E. coli incubation of proteins: elongation PVDV-membranes factor Tu, methionine with anti-TF ab biosynthesis enzyme, followed by isocitrate fluorescence dehydrogenase, labelled secondary glutamine-tRNA- ab synthetase Identification of Pin1 Catalytic domain Sequential electro- 1000 pentapeptides of SPOT (139) inhibitors of human Pin1 blotting to PVDF- general structure Ac- membranes, Xaa-pThr-Yaa-Zaa- incubation of Gln(linker) with Xaa and PVDV-membranes Zaa = Phe, Abz, Bip, with anti-Pin1 ab Bth, Cha, Dbg, Nal, followed by tBuGly, tBuPhe, Thi secondary ab Yaa = MeAla, MePhe, Pip, Pia, 4Pip, Thz, Tic, Pro, Acc, Amb (all possible combinations) Identification of Human Pin1 ProQ Diamond 87 phosphopeptides DIPP (127) substrates subsequent to derived from know phosphatase phosphorylation sites treatment Arginin Methyl-transferases (RMTs) Substrate screening RMT1, CARM1 3H-AdoMet and 36 PABP1 and H3 SPOT (140) tritiumimaging derived peptides containing central Arg Solution phase assay Protein arginine MALDI-TOF/MS GGRGGFGC DIPP (141) with tagged substrates methyl-transferase allowing selective 1 (RMT1) capturing SUMO-Transferases Consensus motif Sumo1 and Hela Anti-Sumo1 ab 10 RanGAP1 derived DIPP & (142) determination cell lysate followed by IgG peptides containing Diff biotin and anti- Sumo consensus motifs streptavidin-HRP and control peptides ab containing R instead of K for assay optimization, peptides covering 11 Sumo targets and negative control and array containing 640 peptide library for consensus motif determination Determination of SUMO kit (Biomol) Anti-Sumo1 ab Overlapping peptide SPOT (143) SUMOylation sites scan of PDE4B2, C2, A4, D5 and alanine scan of putative PDE4D5 sites Ubiquitin-Transferases Determination of Ubiquitination kit Anti-ubiquitin ab Overlapping PDE4D5 SPOT (144) ubiquitinylation target (Biomol) peptide scan, 6 arrestin sites 2 derived peptides Glycosyl-transferases Assay for monitoring ppGalNACT MALDI-TOF/MS GAGAPGPTPGPAGAG DIPP (145) O-glycosylation isoform T2 K, GAGAPGPSPGPAGAG K, GAPGPTPGPAGK, AcPTPGPAGK, GAPGSTAPPAGK, GAHGVTSAPAGK, GAAPDTRPAAGK, YSPTSPSKR, PTTDSTTPAPTTK, DDSIEGSGGR O-glycopeptide library GalNAc-T1 and MALDI-TOF/MS, VTSAPDTRPAPGSTAP DIPP (146) production for disease T2 lectin reactivity and PAHG ± GalNAc, associated mabs 5E5 and 2D9 PTTTPITTTTTTVTPTPT autoantibody GTQTPTTTPISTTC, screening and CPPTPSATTPAPPSSS substrate specificity APPETTAA, comparison LSESTTQLPGGGPGCA , MEELGMAPALQPTQG AMPAF, PRFQDSSSSKAPPPSL PSPSPLPG, WKFEHCNFNDYTTRLR ENEL Glycosylation of GalNAc-T2 and MALDI-TOF/MS Peptides derived from DIPP (147) printed peptides for T4 M. musculus podoplanin peptide diversification for mapping of glycopeptide specific abs Histone Deacetylases (HDACs), Sirtuins (Sirts), ADP-Ribosyl-transferases (ARTs) Detection of ADP- pertussis toxin (B. 32P NAD+ and 5- to 25-mer C-terminal SPOT (148) ribosylation sites pertussis) phosphorimaging peptides of the G-
Protein -subunits of Gi3, Gi, Gs, Go1, Go2, GoX1, Trod, Gz, Gq/11 and Gh, partial substitutional analysis of the 16-mer C-terminal peptide of the
Gi3 -subunit Determination of Pea HDAC Free amino groups Overlapping peptides SPOT (149) substrate specificity complexes HD1 generated by containing KAc from and HD2 (P. HDAC action were histones H3 and H4 sativum) chemically acetylated, detection of 14C- labelled acetyl residues by autoradiography Analysis of the HDAC8 MALDI-TOF/MS Peptides derived from DIPP (150) influence of local and (SAMDI assay) the first 19 aa of H4: distal substrate truncated peptides, R sequences on activity substitutions, KAc and methylated variants, deletion and extensions variants and substitutions Assay for HDAC HDAC1-3, HDAC8 MALDI-TOF/MS 361 Member acetylated DIPP (151) profiling and HDAC3 (SAMDI assay) peptide library: togehter with GXKAcZGC, X, Z ≠ Cys SMRT Detection of ADP- SIR2 (T. brucei) 32P NAD+ and Overlapping peptide DIPP (152) ribosylation sites phosphorimaging scan of B. taurus H1.1 Substrate specificity Human Sirt3 Anti-Sirt3 ab 229 Peptides derived SPOT (153) determination and followed by HRP- from known acetylation identification of novel conjugated sites and 300 lysine- in vivo substrates secondary anti-IgG centered 9meric ab peptides randomly selected from mitochondrial proteome Substrate specificity HDAC8, Sirt1, MALDI-TOF/MS 361 Peptides DIPP (154) determination and HDAC2, (SAMDI assay) GRKAcXZC, X, Z ≠ Cys identification of isoform HDAC3/SMRT, and 54 peptides to specific substrates Hela Jurkat and investigate the function smooth muscle of the Arg residue N- cell lysate and terminally to KAc nuclear Hela lysates treated ± thymidine and nocodazole Identification of in vivo Human Sirt3 Anti-Sirt3 ab 240 peptides derived SPOT (155) substrates followed by HRP- from known conjugated mitochondrial acetylation secondary anti-IgG sites ab Proteases Substrate specificity Chymotrypsin, Fluorescence of 400 dipeptides (156) determination papain released FITC- SPOT labelled peptide fragment Identification of key Pancreatic Chemoluminescenc Substitutional scan of 3 (157) residues in protease elastase (S. e mediated by POD OMTKY3 derived SPOT inhibitors scrofa) and third fused to protease peptides, substitutional domain of turkey analysis and length scan ovomucoid inhibitor (OMTKY3) Substrate specificity Outer membrane Fluorescence Library screen: AGXA, (158) SPOT determination protease T increase by P2RRA, AP1RA, ARP-1A, (OmpT) (E.coli), clavage of ARRP-2 trypsin internally queched Dap(Dnp)/Abz peptides Identification of Cathepsin C, Fluorescence Putative Cys protease (159) protease inhibitors cathepsin L imaging of inhibitors fused to PNA DCSD fluorescein labelled derivatives PNA-peptide conjugates Identification of Purified proteases Protease SPOT 19 Transferrin receptor (160) proteases responsible A80 and A85 assay – derived peptides and SPOT for transferrin receptor (cathepsin G, Fluorescence of substitutions cleavage neutrophil released Abz- elastase) ± anti labelled peptide neutrophil fragment elastase ab (AHN- 10) and inhibitors prefabloc SC, aprotinin, elastatinal, E64, phosphoramidon, pepstatin A, EDTA, DMSO Assay for monitoring of Caspase 3, Jurkat Fluorescence 7 Peptidic acrylates as (161) proteolytic activity cell lysate ± imaging of protease inhibitors fused DCSD granzyme B and fluorescein labelled to PNA derivatives inhibitors PNA-peptide conjugates Substrate cleavage Serine protease Protease SPOT Overlapping peptide (162) site determination DegP (E.coli) assay – scans of PapA SPOT Fluorescence of released Abz- labelled peptide fragment Determination of Trypsin, Fluorescence Ac-Leu-Gly-Pro-Lys- (163) substrate specificity gramzyme B, increase of ACC-linker, Ac-Nle-Thr- DIPP thrombin deacylated amino- Pro-Lys-ACC-linker, and coumarine Ac-Ile-Glu-Pro-Asp- derivative ACC-linker or 361- member library of general structure Ac-Ala- Xaa1-Xaa2-Lys-ACC- linker with Xaa1=Xaa2= all proteinogenic amino acids except cysteine) Protease assay using Thrombin, Fluorescence of Boc-VPR-MCA and 2 (164) microarrays containing chymotrypsin and released methoxy- BODIPY labelled casein SPNS glycerol nanodroplets caspase 2 ± coumarylamine substrates, peptide-MCA and aerosol deposited corresponding substrates: VEID, LEHD, substances CHO-inhibitors, YVAD, DEVD, VDVAD, thrombin ± IETD and 352 benzamidine, compound library plasminogen activator, urokinase, factor Xa, plasmin, kallikrein and caspases 2, 4, 6 for compound library incubation ± VEID-MCA or LEHD-MCA Method for solution- Caspase 3, Fluorescence NleTRP, DEVD and 192 (165) based screening of thrombin, plasmin imaging, member split and mix DCSD protease substrate and apoptotic and rhodamine-labelled tetrapeptide library: specificity non apoptotic cell protease substrate R1R2R3R4, R1 = D, R, L, lysate linked to PNA, R2 = F, V, T, P, R3 = D, R, T, P, R4 = D, R, P, Nle, spatial deconvolution by oligopeptide microarray hybridization Protease activity Lysyl Fluorescence SSSSK-linker-DANSyl, (166) measurement using endopeptidase, increase of SSSSE-linker-DANSyl, Diff semi-wet chymotrypsin, V8 released DANSyl DANSyl-FKSSKS supramolecular protease and derivative hydrogel inhibitors Determination of Glandular Protease SPOT Semenogelin I and II (167) substrate requirements kallikrein 2 (hK2), assay – derived peptides with N- SPOT and development of a trypsin Fluorescence of term. Abz, for the best hK2 avtivated prodrug released Abz- two substrates aa for prostate cancer labelled peptide substitutions in P1’ and therapy fragment Lys or His substitutions in P1 and fluorescence quenched combinatorial library with radom sequences in P3-P1: YIGKAXXX, X ≠ Cys Method using real-time Factor Xa real-time SPR Prothrombin factor Xa (168) SPR imaging to obtain imaging (anti-Flag recognition sequences DIPP multiplexed kinetic ab) CSGIEGRDYKDDDDK, informations and CSGIEGADYKDDDDK, analysis of the CSGDYKDDDDK sequence specificity of factor Xa Substrate specificity APC, kallikrein, Fluorescence 722 Member library: (169) SPNS determination factor VIIa and TF, increase of P4P3P2P1, P4 = Ala, P3, IXa, XIa, XIIa, released amino- P2 ≠ Cys, P1 = Lys, Arg complement C1r, coumarin derivative C1s, complement factor D, trypsin, tryptase, subtilisin carlsberg, cathepsin B, G, H, K, L, S, V, rhodesain, papain, chymopapain, ficain, stem bromelain Substrate specificity Thrombin, Fluorescence 722 Member library: (170) SPNS determination plasmin, factor Xa, increase of P4P3P2P1, P4 = Ala, P3, uPA, thrombin (B. released amino- P2 ≠ Cys, P1 = Lys, Arg taurus, S. coumarin derivative salmothymus) Identification of Kallikrein, factor Fluorescence of Z-FR-MCA, Boc-IEGR- (171) optimized substrates Xa, thrombin, released methoxy- MCA, Boc-VPR-MCA, SPNS for blood proteases uPA, plasmin and coumarylamine glutaryl-GR-MCA, Boc- plasma treated ± VLK-MCA and 361 Ca2+, tissue compound library: Ac- factor, kaolin, uPA XXXX-ACC and 10 plasma samples and 10 proteases (kallikrein, factor Xa, XIIa, XIa, IXa, VIIa, thrombin, APC, uPA, plasmin) Identification of ADAM8 Protease SPOT 34 Peptides derived (172) substrates assay – from proteins involved in SPOT Fluorescence of inflammatory processes released Abz- and immune response in labelled peptide the nervous system and fragment various peptide derived from MBP cleavage sites Analysis of protease Chymopapain, Fluorescence 10000 Member PNA (173) cleavage specificity subtilisin increase by encoded library: DCSD clavage of TAMRA-ßAla-Ser-Xaa4- internally queched Xaa3-Xaa2-Xaa1-Ala- peptides(FAM/TAM Lys(FAM)-Ttds- RA) Lys(PNA)-amide with TAMRA = Quencher/Normalization fluorophor; FAM = Fluorophor, Ttds = PEG- based-linker moiety, Xaa = random positions with amino acid residues Ala, Asp, Phe, Lys, Leu, Asn, Pro, Ser, Val, Tyr. Monitoring caspase Purified caspase 3 Fluorescence ADEVDA and negative (174) activity or lysates of CHO imaging control AEVEEA flanked DIPP cells treated with subsequent to by Cy3 fluorophore and staurosporin protease treatment QSY7-quencher moiety. Positive control without quencher Assay for measuring Caspase 3 and 8, MALDI-TOF/MS DEDVAFC, IETDAFC, (175) caspase activities in SKW6.4 and (SAMDI assay) CGGDEVDSG, DIPP cell lysates Jurkat (caspase 8 CGDEVDSGVDEVA, or deficient) lysate CGKRKGDEVDSG, treated ± CGGIETDSG, LzCD95L or CDGIETDSG, staurosporine CDGIETDSGVDDD, CGELDSGIETDSG Surface optimization Chymotrypsin fluorescence Biotinylated peptides of (176) for SPOT synthesis imaging general structure biotin- SPOT subsequent to GA-P1-G-linker with P1= treatment with Cy5- A,F, G, I, L, P, V, W labelled streptavidin SPR based assay for Caspase 3, 6, 8, Surface plasmon Caspase substrates (177) monitoring protease CHO lysate resonance imaging DEVD, VEID, IETD and DIPP activities incubated ± subsequent to negative controls EVEE, staurosporine and treatment with VIEE, TIEE, inhibitor DEVD- protease followed respectively, flanked by CHO by streptavidin cysteinyl-PEG-linker N- terminally and by GGSK(biotinyl)-amide C- terminally Multiplexed solution- Trypsin, Protease cleavage Over 1000 DNA (178) phase assay for chymotrypsin, separates His-tag encoded, biotinylated DCSD protease profiling endoproteinases from biotin residue peptide derivatives with Asp-N, Glu-C, allowing removal of pentaHis-tag Lys-C, Arg-C, uncleaved peptides representing substrates thrombin, factor by streptavidin for several protease Xa, HRVI, beads, readout groups caspases, MMPs, using anti-His-tag enterokinases, ab tobacco etch virus protease and Jurkat cell lysate incubated ± Fas ab or Bcl2 overexpressing Jurkat lysate Design of biosensors Botulinium FRET activity SNAP-25 peptide (179) neurotoxin and assay yielding substrate Diff light chain of fluorescence (FITC/DABCYL labelled) botulinium increase neurotoxin subsequent to proteolytic cleavage Substrate specificity Subtilase 3 S. Protease SPOT 72 Peptides derived SPOT (180) determination lycopersicum assay – from systemin and Fluorescence of substitution analysis released Abz- using A, F, P, S, N, D, K, labelled peptide V, L, and H for P5 to P3´- fragment position Validation of protease Caspase 3, Fluorescence 10 Peptide sequences DIPP (181) assay caspase 7, increase of derived from known chymotrypsin, deacylated substrates C-terminally subtilisin, rhodamine linked to fluorophore thrombin, trypsin derivative Substrate specificity Granzyme A, Fluorescence 1000 Biotinylated DIPP (182) comparison granzyme K and decrease random peptides and 24 inactive variants subsequent to controls treatment with fluorescein labelled streptavidin Monitoring of Thrombin, Fluorescence Bz-FVR-ACC, Bz-VPR- DIPP (183) proteolytic activities plasmin, increase of ACC, Ac-IEPD-ACC, onto fluorous based chymotrypsin, deacylated Cbz-FR-ACC, Suc-AFK- microarrays trypsin, granzyme aminocoumarine ACC; Suc-AAPF-ACC, B derivative Bz-FVR-AMC, Bz-VPR- AMC, AC-IEPD-AMC, Cbz-FR-AMC, Suc-AFK- AMC, Suc-AAPF-AMC Miscellaneous Assay for hydrolytic Epoxide hydrolase Enzyme recognition Asp and Lys derivatives DIPP (184) enzymes (proteases, (R. rhodochrous), head coupled to esterases, epoxid acetylcholin fluorogenic moiety hydrolases, esterase (E. which serves as a phosphatases) electricus), trypsin reporter group that (B. taurus), AP (B. translates taurus) enzymatic activity into fluorescence readouts Novel based assay for HRP, AP, -Gal Fluorescently 10000 Random peptide DIPP (185) identification of labelled substrate sequences modulators of enzyme analoga function
CLPP = Cross Linking of Pre-synthesized Peptides DCSD = DNA /PNA Chips as Sorting Device Diff. = Different technologies DIPP = Directed Immobilization of Pre-synthesized Peptides DPCC = Deposition of Peptide-Cellulose Conjugates (Celluspots) LDPS = Light Directed Peptide Synthesis SODA = Synthesis On Defined Areas SPNS = Solution Phase and Nano Spray SPOT = SPOT Synthesis of Peptides References 1. Luo, K., Zhou, P. and Lodish, H.F. (1995) The specificity of the transforming growth factor beta receptor kinases determined by a spatially addressable peptide library. Proc Natl Acad Sci U S A, 92, 11761-11765. 2. oomik, R. and Ek, P. (1997) A potent and highly selective peptide substrate for protein kinase C assay. Biochem J, 322 ( Pt 2), 455-460. 3. Moilanen, A.M., Karvonen, U., Poukka, H., Janne, O.A. and Palvimo, J.J. (1998) Activation of androgen receptor function by a novel nuclear protein kinase. Mol Biol Cell, 9, 2527-2543. 4. Dostmann, W.R., Nickl, C., Thiel, S., Tsigelny, I., Frank, R. and Tegge, W.J. (1999) Delineation of selective cyclic GMP-dependent protein kinase Ialpha substrate and inhibitor peptides based on combinatorial peptide libraries on paper. Pharmacol Ther, 82, 373-387. 5. Himpel, S., Tegge, W., Frank, R., Leder, S., Joost, H.G. and Becker, W. (2000) Specificity determinants of substrate recognition by the protein kinase DYRK1A. J Biol Chem, 275, 2431- 2438. 6. Loog, M., Toomik, R., Sak, K., Muszynska, G., Jarv, J. and Ek, P. (2000) Peptide phosphorylation by calcium-dependent protein kinase from maize seedlings. Eur J Biochem, 267, 337-343. 7. Dostmann, W.R., Tegge, W., Frank, R., Nickl, C.K., Taylor, M.S. and Brayden, J.E. (2002) Exploring the mechanisms of vascular smooth muscle tone with highly specific, membrane- permeable inhibitors of cyclic GMP-dependent protein kinase Ialpha. Pharmacol Ther, 93, 203- 215. 8. Lizcano, J.M., Deak, M., Morrice, N., Kieloch, A., Hastie, C.J., Dong, L., Schutkowski, M., Reimer, U. and Alessi, D.R. (2002) Molecular basis for the substrate specificity of NIMA-related kinase-6 (NEK6). Evidence that NEK6 does not phosphorylate the hydrophobic motif of ribosomal S6 protein kinase and serum- and glucocorticoid-induced protein kinase in vivo. J Biol Chem, 277, 27839-27849. 9. Uttamchandani, M., Chan, E.W., Chen, G.Y. and Yao, S.Q. (2003) Combinatorial peptide microarrays for the rapid determination of kinase specificity. Bioorg Med Chem Lett, 13, 2997- 3000. 10. Panse, S., Dong, L., Burian, A., Carus, R., Schutkowski, M., Reimer, U. and Schneider-Mergener, J. (2004) Profiling of generic anti-phosphopeptide antibodies and kinases with peptide microarrays using radioactive and fluorescence-based assays. Mol Divers, 8, 291-299. 11. Rychlewski, L., Kschischo, M., Dong, L., Schutkowski, M. and Reimer, U. (2004) Target specificity analysis of the Abl kinase using peptide microarray data. J Mol Biol, 336, 307-311. 12. Bucko-Justyna, M., Lipinski, L., Burgering, B.M. and Trzeciak, L. (2005) Characterization of testis- specific serine-threonine kinase 3 and its activation by phosphoinositide-dependent kinase-1- dependent signalling. FEBS J, 272, 6310-6323. 13. Wang, H. and Brautigan, D.L. (2006) Peptide microarray analysis of substrate specificity of the transmembrane Ser/Thr kinase KPI-2 reveals reactivity with cystic fibrosis transmembrane conductance regulator and phosphorylase. Mol Cell Proteomics, 5, 2124-2130. 14. Papadopoulos, C., Arato, K., Lilienthal, E., Zerweck, J., Schutkowski, M., Chatain, N., Muller- Newen, G., Becker, W. and de la Luna, S. (2010) Splice variants of the dual-specificity tyrosine phosphorylation-regulated kinase 4 (DYRK4) differ in their subcellular localization and catalytic activity. J Biol Chem. 15. Beullens, M., Vancauwenbergh, S., Morrice, N., Derua, R., Ceulemans, H., Waelkens, E. and Bollen, M. (2005) Substrate specificity and activity regulation of protein kinase MELK. J Biol Chem, 280, 40003-40011. 16. Mah, A.S., Elia, A.E., Devgan, G., Ptacek, J., Schutkowski, M., Snyder, M., Yaffe, M.B. and Deshaies, R.J. (2005) Substrate specificity analysis of protein kinase complex Dbf2-Mob1 by peptide library and proteome array screening. BMC Biochem, 6, 22. 17. Pouchain, D., Diaz-Mochon, J.J., Bialy, L. and Bradley, M. (2007) A 10,000 member PNA- encoded peptide library for profiling tyrosine kinases. ACS Chem Biol, 2, 810-818. 18. Stulemeijer, I.J., Stratmann, J.W. and Joosten, M.H. (2007) Tomato mitogen-activated protein kinases LeMPK1, LeMPK2, and LeMPK3 are activated during the Cf-4/Avr4-induced hypersensitive response and have distinct phosphorylation specificities. Plant Physiol, 144, 1481- 1494. 19. Leung, G.C., Ho, C.S., Blasutig, I.M., Murphy, J.M. and Sicheri, F. (2007) Determination of the Plk4/Sak consensus phosphorylation motif using peptide spots arrays. FEBS Lett, 581, 77-83. 20. Warner, N., Wybenga-Groot, L.E. and Pawson, T. (2008) Analysis of EphA4 receptor tyrosine kinase substrate specificity using peptide-based arrays. FEBS J, 275, 2561-2573. 21 Vilk, G., Weber, J.E., Turowec, J.P., Duncan, J.S., Wu, C., Derksen, D.R., Zien, P., Sarno, S., Donella-Deana, A., Lajoie, G. et al. (2008) Protein kinase CK2 catalyzes tyrosine phosphorylation in mammalian cells. Cell Signal, 20, 1942-1951. 22 Merckx, A., Echalier, A., Langford, K., Sicard, A., Langsley, G., Joore, J., Doerig, C., Noble, M. and Endicott, J. (2008) Structures of P. falciparum protein kinase 7 identify an activation motif and leads for inhibitor design. Structure, 16, 228-238. 23 Parikh, K., Diks, S.H., Tuynman, J.H., Verhaar, A., Lowenberg, M., Hommes, D.W., Joore, J., Pandey, A. and Peppelenbosch, M.P. (2009) Comparison of peptide array substrate phosphorylation of c-Raf and mitogen activated protein kinase kinase kinase 8. PLoS One, 4, e6440. 24 Bohmer, F.D. and Uecker, A. (2009) A substrate peptide for the FLT3 receptor tyrosine kinase. Br J Haematol, 144, 127-130. 25 Han, X., Sonoda, T., Mori, T., Yamanouchi, G., Yamaji, T., Shigaki, S., Niidome, T. and Katayama, Y. (2010) Protein kinase substrate profiling with a high-density peptide microarray. Comb Chem High Throughput Screen, 13, 777-789. 26 Miller, M., Donat, S., Rakette, S., Stehle, T., Kouwen, T.R., Diks, S.H., Dreisbach, A., Reilman, E., Gronau, K., Becher, D. et al. (2010) Staphylococcal PknB as the First Prokaryotic Representative of the Proline-Directed Kinases. PLoS One, 5, e9057. 27 Fraser, J.A., Vojtesek, B. and Hupp, T.R. (2010) A Novel p53 Phosphorylation Site within the MDM2 Ubiquitination Signal: I. PHOSPHORYLATION AT SER269 IN VIVO IS LINKED TO INACTIVATION OF p53 FUNCTION. J Biol Chem, 285, 37762-37772. 28 Galello, F., Portela, P., Moreno, S. and Rossi, S. (2010) Characterization of substrates that have a differential effect on Saccharomyces cerevisiae protein kinase A holoenzyme activation. J Biol Chem, 285, 29770-29779. 29 Szallasi, Z., Denning, M.F., Chang, E.Y., Rivera, J., Yuspa, S.H., Lehel, C., Olah, Z., Anderson, W.B. and Blumberg, P.M. (1995) Development of a rapid approach to identification of tyrosine phosphorylation sites: application to PKC delta phosphorylated upon activation of the high affinity receptor for IgE in rat basophilic leukemia cells. Biochem Biophys Res Commun, 214, 888-894. 30 Edlund, M., Wikstrom, K., Toomik, R., Ek, P. and Obrink, B. (1998) Characterization of protein kinase C-mediated phosphorylation of the short cytoplasmic domain isoform of C- CAM. FEBS Lett, 425, 166-170. 31 Buss, H., Dorrie, A., Schmitz, M.L., Frank, R., Livingstone, M., Resch, K. and Kracht, M. (2004) Phosphorylation of serine 468 by GSK-3beta negatively regulates basal p65 NF- kappaB activity. J Biol Chem, 279, 49571-49574. 32 Carnegie, G.K., Smith, F.D., McConnachie, G., Langeberg, L.K. and Scott, J.D. (2004) AKAP- Lbc nucleates a protein kinase D activation scaffold. Mol Cell, 15, 889-899. 33 Li, Y., Keller, D.M., Scott, J.D. and Lu, H. (2005) CK2 phosphorylates SSRP1 and inhibits its DNA-binding activity. J Biol Chem, 280, 11869-11875. 34 Collins, M.O., Yu, L., Coba, M.P., Husi, H., Campuzano, I., Blackstock, W.P., Choudhary, J.S. and Grant, S.G. (2005) Proteomic analysis of in vivo phosphorylated synaptic proteins. J Biol Chem, 280, 5972-5982. 35 Wiesner, S., Wybenga-Groot, L.E., Warner, N., Lin, H., Pawson, T., Forman-Kay, J.D. and Sicheri, F. (2006) A change in conformational dynamics underlies the activation of Eph receptor tyrosine kinases. EMBO J, 25, 4686-4696. 36 Delgado, J.Y., Coba, M., Anderson, C.N., Thompson, K.R., Gray, E.E., Heusner, C.L., Martin, K.C., Grant, S.G. and O'Dell, T.J. (2007) NMDA receptor activation dephosphorylates AMPA receptor glutamate receptor 1 subunits at threonine 840. J Neurosci, 27, 13210-13221. 37 Amanchy, R., Zhong, J., Molina, H., Chaerkady, R., Iwahori, A., Kalume, D.E., Gronborg, M., Joore, J., Cope, L. and Pandey, A. (2008) Identification of c-Src tyrosine kinase substrates using mass spectrometry and peptide microarrays. J Proteome Res, 7, 3900-3910. 38 Yu, J.C., Chen, J.R., Lin, C.H., Zhang, G., Lam, P.S., Wenger, K.H., Mozaffari, F.B., Huang, S.T. and Borke, J.L. (2009) Tensile strain-induced Ets-2 phosphorylation by CaMKII and the homeostasis of cranial sutures. Plast Reconstr Surg, 123, 83S-93S. 39 Santamaria, A., Wang, B., Elowe, S., Malik, R., Zhang, F., Bauer, M., Schmidt, A., Sillje, H.H., Koerner, R. and Nigg, E.A. The Plk1-dependent phosphoproteome of the early mitotic spindle. Mol Cell Proteomics. 40 Wanichawan, P., Louch, W.E., Hortemo, K.H., Austbo, B., Lunde, P.K., Scott, J.D., Sejersted, O.M. and Carlson, C.R. (2011) Full length cardiac Na+-Ca2+ exchanger (NCX1) protein is not phosphorylated by protein kinase A (PKA). Am J Physiol Cell Physiol. 41 Diks, S.H., Kok, K., O'Toole, T., Hommes, D.W., van Dijken, P., Joore, J. and Peppelenbosch, M.P. (2004) Kinome profiling for studying lipopolysaccharide signal transduction in human peripheral blood mononuclear cells. J Biol Chem, 279, 49206-49213. 42 Hayashi, M., Fearns, C., Eliceiri, B., Yang, Y. and Lee, J.D. (2005) Big mitogen-activated protein kinase 1/extracellular signal-regulated kinase 5 signaling pathway is essential for tumor-associated angiogenesis. Cancer Res, 65, 7699-7706. 43 Lowenberg, M., Tuynman, J., Bilderbeek, J., Gaber, T., Buttgereit, F., van Deventer, S., Peppelenbosch, M. and Hommes, D. (2005) Rapid immunosuppressive effects of glucocorticoids mediated through Lck and Fyn. Blood, 106, 1703-1710. 44 van Baal, J.W., Diks, S.H., Wanders, R.J., Rygiel, A.M., Milano, F., Joore, J., Bergman, J.J., Peppelenbosch, M.P. and Krishnadath, K.K. (2006) Comparison of kinome profiles of Barrett's esophagus with normal squamous esophagus and normal gastric cardia. Cancer Res, 66, 11605-11612. 45 Lowenberg, M., Tuynman, J., Scheffer, M., Verhaar, A., Vermeulen, L., van Deventer, S., Hommes, D. and Peppelenbosch, M. (2006) Kinome analysis reveals nongenomic glucocorticoid receptor-dependent inhibition of insulin signaling. Endocrinology, 147, 3555- 3562. 46 Shigaki, S., Yamaji, T., Han, X., Yamanouchi, G., Sonoda, T., Okitsu, O., Mori, T., Niidome, T. and Katayama, Y. (2007) A peptide microarray for the detection of protein kinase activity in cell lysate. Anal Sci, 23, 271-275. 47 Ritsema, T., Joore, J., van Workum, W. and Pieterse, C.M. (2007) Kinome profiling of Arabidopsis using arrays of kinase consensus substrates. Plant Methods, 3, 3. 48 de Borst, M.H., Diks, S.H., Bolbrinker, J., Schellings, M.W., van Dalen, M.B., Peppelenbosch, M.P., Kreutz, R., Pinto, Y.M., Navis, G. and van Goor, H. (2007) Profiling of the renal kinome: a novel tool to identify protein kinases involved in angiotensin II-dependent hypertensive renal damage. Am J Physiol Renal Physiol, 293, F428-437. 49 Lemeer, S., Jopling, C., Naji, F., Ruijtenbeek, R., Slijper, M., Heck, A.J. and den Hertog, J. (2007) Protein-tyrosine kinase activity profiling in knock down zebrafish embryos. PLoS ONE, 2, e581. 50 Lemeer, S., Ruijtenbeek, R., Pinkse, M.W., Jopling, C., Heck, A.J., den Hertog, J. and Slijper, M. (2007) Endogenous phosphotyrosine signaling in zebrafish embryos. Mol Cell Proteomics, 6, 2088-2099. 51 Diks, S.H., Parikh, K., van der Sijde, M., Joore, J., Ritsema, T. and Peppelenbosch, M.P. (2007) Evidence for a minimal eukaryotic phosphoproteome? PLoS ONE, 2, e777. 52 Bowick, G.C., Fennewald, S.M., Scott, E.P., Zhang, L., Elsom, B.L., Aronson, J.F., Spratt, H.M., Luxon, B.A., Gorenstein, D.G. and Herzog, N.K. (2007) Identification of differentially activated cell-signaling networks associated with pichinde virus pathogenesis by using systems kinomics. J Virol, 81, 1923-1933. 53 de la Fuente van Bentem, S., Anrather, D., Dohnal, I., Roitinger, E., Csaszar, E., Joore, J., Buijnink, J., Carreri, A., Forzani, C., Lorkovic, Z.J. et al. (2008) Site-specific phosphorylation profiling of Arabidopsis proteins by mass spectrometry and peptide chip analysis. J Proteome Res, 7, 2458-2470. 54 Mori, T., Inamori, K., Inoue, Y., Han, X., Yamanouchi, G., Niidome, T. and Katayama, Y. (2008) Evaluation of protein kinase activities of cell lysates using peptide microarrays based on surface plasmon resonance imaging. Anal Biochem, 375, 223-231. 55 Neuman, B.W., Joseph, J.S., Saikatendu, K.S., Serrano, P., Chatterjee, A., Johnson, M.A., Liao, L., Klaus, J.P., Yates, J.R., 3rd, Wuthrich, K. et al. (2008) Proteomics analysis unravels the functional repertoire of coronavirus nonstructural protein 3. J Virol, 82, 5279-5294. 56 De Keersmaecker, K., Versele, M., Cools, J., Superti-Furga, G. and Hantschel, O. (2008) Intrinsic differences between the catalytic properties of the oncogenic NUP214-ABL1 and BCR-ABL1 fusion protein kinases. Leukemia, 22, 2208-2216. 57 Jinnin, M., Medici, D., Park, L., Limaye, N., Liu, Y., Boscolo, E., Bischoff, J., Vikkula, M., Boye, E. and Olsen, B.R. (2008) Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat Med, 14, 1236-1246. 58 Tuynman, J.B., Vermeulen, L., Boon, E.M., Kemper, K., Zwinderman, A.H., Peppelenbosch, M.P. and Richel, D.J. (2008) Cyclooxygenase-2 inhibition inhibits c-Met kinase activity and Wnt activity in colon cancer. Cancer Res, 68, 1213-1220. 59 Bratland, A., Boender, P.J., Hoifodt, H.K., Ostensen, I.H., Ruijtenbeek, R., Wang, M.Y., Berg, J.P., Lilleby, W., Fodstad, O. and Ree, A.H. (2009) Osteoblast-induced EGFR/ERBB2 signaling in androgen-sensitive prostate carcinoma cells characterized by multiplex kinase activity profiling. Clin Exp Metastasis, 26, 485-496. 60 Coba, M.P., Pocklington, A.J., Collins, M.O., Kopanitsa, M.V., Uren, R.T., Swamy, S., Croning, M.D., Choudhary, J.S. and Grant, S.G. (2009) Neurotransmitters drive combinatorial multistate postsynaptic density networks. Sci Signal, 2, ra19. 61 Schrage, Y.M., Briaire-de Bruijn, I.H., de Miranda, N.F., van Oosterwijk, J., Taminiau, A.H., van Wezel, T., Hogendoorn, P.C. and Bovee, J.V. (2009) Kinome profiling of chondrosarcoma reveals SRC-pathway activity and dasatinib as option for treatment. Cancer Res, 69, 6216-6222. 62 Ritsema, T., Brodmann, D., Diks, S.H., Bos, C.L., Nagaraj, V., Pieterse, C.M., Boller, T., Wiemken, A. and Peppelenbosch, M.P. (2009) Are small GTPases signal hubs in sugar- mediated induction of fructan biosynthesis? PLoS One, 4, e6605. 63 Maat, W., el Filali, M., Dirks-Mulder, A., Luyten, G.P., Gruis, N.A., Desjardins, L., Boender, P., Jager, M.J. and van der Velden, P.A. (2009) Episodic Src activation in uveal melanoma revealed by kinase activity profiling. Br J Cancer, 101, 312-319. 64 Ritsema, T. and Peppelenbosch, M.P. (2009) Kinome profiling of sugar signaling in plants using multiple platforms. Plant Signal Behav, 4, 1169-1173. 65 Han, X., Yamanouchi, G., Mori, T., Kang, J.H., Niidome, T. and Katayama, Y. (2009) Monitoring protein kinase activity in cell lysates using a high-density peptide microarray. J Biomol Screen, 14, 256-262. 66 Sikkema, A.H., Diks, S.H., den Dunnen, W.F., ter Elst, A., Scherpen, F.J., Hoving, E.W., Ruijtenbeek, R., Boender, P.J., de Wijn, R., Kamps, W.A. et al. (2009) Kinome profiling in pediatric brain tumors as a new approach for target discovery. Cancer Res, 69, 5987-5995. 67 Jalal, S., Arsenault, R., Potter, A.A., Babiuk, L.A., Griebel, P.J. and Napper, S. (2009) Genome to kinome: species-specific peptide arrays for kinome analysis. Sci Signal, 2, pl1. 68 Ter Elst, A., Diks, S.H., Kampen, K.R., Hoogerbrugge, P.M., Ruijtenbeek, R., Boender, P.J., Sikkema, A.H., Scherpen, F.J., Kamps, W.A., Peppelenbosch, M.P. et al. (2010) Identification of new possible targets for leukemia treatment by kinase activity profiling. Leuk Lymphoma. 69 Taher, T.E., Parikh, K., Flores-Borja, F., Mletzko, S., Isenberg, D.A., Peppelenbosch, M.P. and Mageed, R.A. (2010) Protein phosphorylation and kinome profiling reveal altered regulation of multiple signaling pathways in B lymphocytes from patients with systemic lupus erythematosus. Arthritis Rheum, 62, 2412-2423. 70 Milani, R., Ferreira, C.V., Granjeiro, J.M., Paredes-Gamero, E.J., Silva, R.A., Justo, G.Z., Nader, H.B., Galembeck, E., Peppelenbosch, M.P., Aoyama, H. et al. (2010) Phosphoproteome reveals an atlas of protein signaling networks during osteoblast adhesion. J Cell Biochem. 71 Willems, S.M., Schrage, Y.M., Bruijn, I.H., Szuhai, K., Hogendoorn, P.C. and Bovee, J.V. (2010) Kinome profiling of myxoid liposarcoma reveals NF-kappaB-pathway kinase activity and casein kinase II inhibition as a potential treatment option. Mol Cancer, 9, 257. 72 Ritsema, T., van Zanten, M., Leon-Reyes, A., Voesenek, L.A., Millenaar, F.F., Pieterse, C.M. and Peeters, A.J. (2010) Kinome Profiling Reveals an Interaction Between Jasmonate, Salicylate and Light Control of Hyponastic Petiole Growth in Arabidopsis thaliana. PLoS One, 5, e14255. 73 Folkvord, S., Flatmark, K., Dueland, S., de Wijn, R., Groholt, K.K., Hole, K.H., Nesland, J.M., Ruijtenbeek, R., Boender, P.J., Johansen, M. et al. (2010) Prediction of response to preoperative chemoradiotherapy in rectal cancer by multiplex kinase activity profiling. Int J Radiat Oncol Biol Phys, 78, 555-562. 74 Vivanco, I., Rohle, D., Versele, M., Iwanami, A., Kuga, D., Oldrini, B., Tanaka, K., Dang, J., Kubek, S., Palaskas, N. et al. (2010) The phosphatase and tensin homolog regulates epidermal growth factor receptor (EGFR) inhibitor response by targeting EGFR for degradation. Proc Natl Acad Sci U S A, 107, 6459-6464. 75 Sikkema, A.H., de Bont, E.S., Molema, G., Dimberg, A., Zwiers, P.J., Diks, S.H., Hoving, E.W., Kamps, W.A., Peppelenbosch, M.P. and den Dunnen, W.F. (2011) VEGFR-2 signalling activity in paediatric pilocytic astrocytoma is restricted to tumour endothelial cells. Neuropathol Appl Neurobiol. 76 Mukhija, S., Germeroth, L., Schneider-Mergener, J. and Erni, B. (1998) Identification of peptides inhibiting enzyme I of the bacterial phosphotransferase system using combinatorial cellulose-bound peptide libraries. Eur J Biochem, 254, 433-438. 77 Dostmann, W.R., Taylor, M.S., Nickl, C.K., Brayden, J.E., Frank, R. and Tegge, W.J. (2000) Highly specific, membrane-permeant peptide blockers of cGMP-dependent protein kinase Ialpha inhibit NO-induced cerebral dilation. Proc Natl Acad Sci U S A, 97, 14772-14777. 78 Houseman, B.T., Huh, J.H., Kron, S.J. and Mrksich, M. (2002) Peptide chips for the quantitative evaluation of protein kinase activity. Nat Biotechnol, 20, 270-274. 79 Rothmeier, A.S., Ischenko, I., Joore, J., Garczarczyk, D., Furst, R., Bruns, C.J., Vollmar, A.M. and Zahler, S. (2009) Investigation of the marine compound spongistatin 1 links the inhibition of PKCalpha translocation to nonmitotic effects of tubulin antagonism in angiogenesis. FASEB J, 23, 1127-1137. 80 Versele, M., Talloen, W., Rockx, C., Geerts, T., Janssen, B., Lavrijssen, T., King, P., Gohlmann, H.W., Page, M. and Perera, T. (2009) Response prediction to a multitargeted kinase inhibitor in cancer cell lines and xenograft tumors using high-content tyrosine peptide arrays with a kinetic readout. Mol Cancer Ther, 8, 1846-1855. 81 Roorda, B.D., Ter Elst, A., Diks, S.H., Meeuwsen-de Boer, T.G., Kamps, W.A. and de Bont, E.S. (2009) PTK787/ZK 222584 inhibits tumor growth promoting mesenchymal stem cells: kinase activity profiling as powerful tool in functional studies. Cancer Biol Ther, 8, 1239-1248. 82 Olaussen, K.A., Commo, F., Tailler, M., Lacroix, L., Vitale, I., Raza, S.Q., Richon, C., Dessen, P., Lazar, V., Soria, J.C. et al. (2009) Synergistic proapoptotic effects of the two tyrosine kinase inhibitors pazopanib and lapatinib on multiple carcinoma cell lines. Oncogene, 28, 4249-4260. 83 Parikh, K., Poppema, S., Peppelenbosch, M.P. and Visser, L. (2009) Extracellular ligation- dependent CD45RB enzymatic activity negatively regulates lipid raft signal transduction. Blood, 113, 594-603. 84 Ghosh, G., Yan, X., Lee, A.G., Kron, S.J. and Palecek, S.P. (2010) Quantifying the sensitivities of EGF receptor (EGFR) tyrosine kinase inhibitors in drug resistant non-small cell lung cancer (NSCLC) cells using hydrogel-based peptide array. Biosens Bioelectron, 26, 424- 431. 85 Schmerwitz, U.K., Sass, G., Khandoga, A.G., Joore, J., Mayer, B.A., Berberich, N., Totzke, F., Krombach, F., Tiegs, G., Zahler, S. et al. (2011) Flavopiridol Protects Against Inflammation by Attenuating Leukocyte-Endothelial Interaction via Inhibition of Cyclin- Dependent Kinase 9. Arterioscler Thromb Vasc Biol, 31, 280-288. 86 Tegge, W., Frank, R., Hofmann, F. and Dostmann, W.R. (1995) Determination of cyclic nucleotide-dependent protein kinase substrate specificity by the use of peptide libraries on cellulose paper. Biochemistry, 34, 10569-10577. 87 Toomik, R., Edlund, M., Ek, P., Obrink, B. and Engstrom, L. (1996) Simultaneously synthesized peptides on continuous cellulose membranes as substrates for protein kinases. Pept Res, 9, 6-11. 88 Tegge, W.J. and Frank, R. (1998) Analysis of protein kinase substrate specificity by the use of peptide libraries on cellulose paper (SPOT-method). Methods Mol Biol, 87, 99-106. 89 MacBeath, G. and Schreiber, S.L. (2000) Printing proteins as microarrays for high-throughput function determination. Science, 289, 1760-1763. 90 Falsey, J.R., Renil, M., Park, S., Li, S. and Lam, K.S. (2001) Peptide and small molecule microarray for high throughput cell adhesion and functional assays. Bioconjug Chem, 12, 346-353. 91 Lesaicherre, M.L., Uttamchandani, M., Chen, G.Y. and Yao, S.Q. (2002) Antibody-based fluorescence detection of kinase activity on a peptide array. Bioorg Med Chem Lett, 12, 2085- 2088. 92 Espanel, X., Walchli, S., Ruckle, T., Harrenga, A., Huguenin-Reggiani, M. and Hooft van Huijsduijnen, R. (2003) Mapping of synergistic components of weakly interacting protein- protein motifs using arrays of paired peptides. J Biol Chem, 278, 15162-15167. 93 Martin, K., Steinberg, T.H., Goodman, T., Schulenberg, B., Kilgore, J.A., Gee, K.R., Beechem, J.M. and Patton, W.F. (2003) Strategies and solid-phase formats for the analysis of protein and peptide phosphorylation employing a novel fluorescent phosphorylation sensor dye. Comb Chem High Throughput Screen, 6, 331-339. 94 Martin, K., Steinberg, T.H., Cooley, L.A., Gee, K.R., Beechem, J.M. and Patton, W.F. (2003) Quantitative analysis of protein phosphorylation status and protein kinase activity on microarrays using a novel fluorescent phosphorylation sensor dye. Proteomics, 3, 1244-1255. 95 Schutkowski, M., Reimer, U., Panse, S., Dong, L., Lizcano, J.M., Alessi, D.R. and Schneider- Mergener, J. (2004) High-content peptide microarrays for deciphering kinase specificity and biology. Angew Chem Int Ed Engl, 43, 2671-2674. 96 Min, D.H., Su, J. and Mrksich, M. (2004) Profiling kinase activities by using a peptide chip and mass spectrometry. Angew Chem Int Ed Engl, 43, 5973-5977. 97 Wang, Z., Lee, J., Cossins, A.R. and Brust, M. (2005) Microarray-based detection of protein binding and functionality by gold nanoparticle probes. Anal Chem, 77, 5770-5774. 98 Su, J., Bringer, M.R., Ismagilov, R.F. and Mrksich, M. (2005) Combining microfluidic networks and peptide arrays for multi-enzyme assays. J Am Chem Soc, 127, 7280-7281. 99 Inamori, K., Kyo, M., Nishiya, Y., Inoue, Y., Sonoda, T., Kinoshita, E., Koike, T. and Katayama, Y. (2005) Detection and quantification of on-chip phosphorylated peptides by surface plasmon resonance imaging techniques using a phosphate capture molecule. Anal Chem, 77, 3979-3985. 100 Rupcich, N., Green, J.R. and Brennan, J.D. (2005) Nanovolume kinase inhibition assay using a sol-gel-derived multicomponent microarray. Anal Chem, 77, 8013-8019. 101 Horiuchi, K.Y., Wang, Y., Diamond, S.L. and Ma, H. (2006) Microarrays for the functional analysis of the chemical-kinase interactome. J Biomol Screen, 11, 48-56. 102 Horiuchi, K.Y., Wang, Y., Diamond, S.L. and Ma, H. (2006) Microarrays for the functional analysis of the chemical-kinase interactome. J Biomol Screen, 11, 48-56. 103 Sun, L., Liu, D. and Wang, Z. (2007) Microarray-based kinase inhibition assay by gold nanoparticle probes. Anal Chem, 79, 773-777. 104 Shults, M.D., Kozlov, I.A., Nelson, N., Kermani, B.G., Melnyk, P.C., Shevchenko, V., Srinivasan, A., Musmacker, J., Hachmann, J.P., Barker, D.L. et al. (2007) A multiplexed protein kinase assay. Chembiochem, 8, 933-942. 105 Akita, S., Umezawa, N., Kato, N. and Higuchi, T. (2008) Array-based fluorescence assay for serine/threonine kinases using specific chemical reaction. Bioorg Med Chem, 16, 7788-7794. 106 Thiele, A., Zerweck, J., Weiwad, M., Fischer, G. and Schutkowski, M. (2009) High-density Peptide microarrays for reliable identification of phosphorylation sites and upstream kinases. Methods Mol Biol, 570, 203-219. 107 Parikh, K., Peppelenbosch, M.P. and Ritsema, T. (2009) Kinome profiling using peptide arrays in eukaryotic cells. Methods Mol Biol, 527, 269-280, x. 108 Hilhorst, R., Houkes, L., van den Berg, A. and Ruijtenbeek, R. (2009) Peptide microarrays for detailed, high-throughput substrate identification, kinetic characterization, and inhibition studies on protein kinase A. Anal Biochem, 387, 150-161. 109 Poot, A.J., van Ameijde, J., Slijper, M., van den Berg, A., Hilhorst, R., Ruijtenbeek, R., Rijkers, D.T. and Liskamp, R.M. (2009) Development of selective bisubstrate-based inhibitors against protein kinase C (PKC) isozymes by using dynamic peptide microarrays. Chembiochem, 10, 2042-2051. 110 Li, T., Liu, D. and Wang, Z. (2009) Microarray-based Raman spectroscopic assay for kinase inhibition by gold nanoparticle probes. Biosens Bioelectron, 24, 3335-3339. 111 Lee, D.W., Kim, H.J., Choi, C.H., Shin, J.H. and Kim, E.K. (2010) Development of a Protein Chip to Measure PKCbeta Activity. Appl Biochem Biotechnol. 112 Han, X. and Katayama, Y. (2010) A Peptide microarray for detecting protein kinase activity in cell lysates. Methods Mol Biol, 669, 183-194. 113 Thiele, A., Weiwad, M., Zerweck, J., Fischer, G. and Schutkowski, M. (2010) High density peptide microarrays for proteome-wide fingerprinting of kinase activities in cell lysates. Methods Mol Biol, 669, 173-181. 114 Lesaicherre, M.L., Uttamchandani, M., Chen, G.Y. and Yao, S.Q. (2002) Developing site- specific immobilization strategies of peptides in a microarray. Bioorg Med Chem Lett, 12, 2079-2083. 115 Reimer, U., Reineke, U. and Schneider-Mergener, J. (2002) Peptide arrays: from macro to micro. Curr Opin Biotechnol, 13, 315-320. 116 Uttamchandani, M., Chen, G.Y., Lesaicherre, M.L. and Yao, S.Q. (2004) Site-specific peptide immobilization strategies for the rapid detection of kinase activity on microarrays. Methods Mol Biol, 264, 191-204. 117 Rodriguez, M., Li, S.S., Harper, J.W. and Songyang, Z. (2004) An oriented peptide array library (OPAL) strategy to study protein-protein interactions. J Biol Chem, 279, 8802-8807. 118 Lee, S.J. and Lee, S.Y. (2004) Microarrays of peptides elevated on the protein layer for efficient protein kinase assay. Anal Biochem, 330, 311-316. 119 Kimura, N., Okegawa, T., Yamazaki, K. and Matsuoka, K. (2007) Site-specific, covalent attachment of poly(dT)-modified peptides to solid surfaces for microarrays. Bioconjug Chem, 18, 1778-1785. 120 Kerman, K., Chikae, M., Yamamura, S. and Tamiya, E. (2007) Gold nanoparticle-based electrochemical detection of protein phosphorylation. Anal Chim Acta, 588, 26-33. 121 Inamori, K., Kyo, M., Matsukawa, K., Inoue, Y., Sonoda, T., Tatematsu, K., Tanizawa, K., Mori, T. and Katayama, Y. (2008) Optimal surface chemistry for peptide immobilization in on- chip phosphorylation analysis. Anal Chem, 80, 643-650. 122 Wong, E.Y. and Diamond, S.L. (2008) Enzyme microarrays assembled by acoustic dispensing technology. Anal Biochem, 381, 101-106. 123 Espanel, X., Huguenin-Reggiani, M. and Hooft van Huijsduijnen, R. (2002) The SPOT technique as a tool for studying protein tyrosine phosphatase substrate specificities. Protein Sci, 11, 2326-2334. 124 Pasquali, C., Curchod, M.L., Walchli, S., Espanel, X., Guerrier, M., Arigoni, F., Strous, G. and Van Huijsduijnen, R.H. (2003) Identification of protein tyrosine phosphatases with specificity for the ligand-activated growth hormone receptor. Mol Endocrinol, 17, 2228-2239. 125 Espanel, X. and Hooft van Huijsduijnen, R. (2005) Applying the SPOT peptide synthesis procedure to the study of protein tyrosine phosphatase substrate specificity: probing for the heavenly match in vitro. Methods, 35, 64-72. 126 Kohn, M., Gutierrez-Rodriguez, M., Jonkheijm, P., Wetzel, S., Wacker, R., Schroeder, H., Prinz, H., Niemeyer, C.M., Breinbauer, R., Szedlacsek, S.E. et al. (2007) A microarray strategy for mapping the substrate specificity of protein tyrosine phosphatase. Angew Chem Int Ed Engl, 46, 7700-7703. 127 Sun, H., Lu, C.H., Uttamchandani, M., Xia, Y., Liou, Y.C. and Yao, S.Q. (2008) Peptide microarray for high-throughput determination of phosphatase specificity and biology. Angew Chem Int Ed Engl, 47, 1698-1702. 128 Govindaraju, T., Jonkheijm, P., Gogolin, L., Schroeder, H., Becker, C.F., Niemeyer, C.M. and Waldmann, H. (2008) Surface immobilization of biomolecules by click sulfonamide reaction. Chem Commun (Camb), 3723-3725. 129 Sacco, F., Tinti, M., Palma, A., Ferrari, E., Nardozza, A.P., Hooft van Huijsduijnen, R., Takahashi, T., Castagnoli, L. and Cesareni, G. (2009) Tumor suppressor density-enhanced phosphatase-1 (DEP-1) inhibits the RAS pathway by direct dephosphorylation of ERK1/2 kinases. J Biol Chem. 130 Sun, H., Tan, L.P., Gao, L. and Yao, S.Q. (2009) High-throughput screening of catalytically inactive mutants of protein tyrosine phosphatases (PTPs) in a phosphopeptide microarray. Chem Commun (Camb), 677-679. 131 Gao, L., Sun, H. and Yao, S.Q. (2010) Activity-based high-throughput determination of PTPs substrate specificity using a phosphopeptide microarray. Biopolymers, 94, 810-819. 132 Ferrari, E., Tinti, M., Costa, S., Corallino, S., Nardozza, A.P., Chatr-Aryamontri, A., Ceol, A., Cesareni, G. and Castagnoli, L. (2010) Identification of new substrates of the protein tyrosine phosphatase PTP1B by bayesian integration of proteome evidence. J Biol Chem. 133 Rathert, P., Dhayalan, A., Murakami, M., Zhang, X., Tamas, R., Jurkowska, R., Komatsu, Y., Shinkai, Y., Cheng, X. and Jeltsch, A. (2008) Protein lysine methyltransferase G9a acts on non-histone targets. Nat Chem Biol, 4, 344-346. 134 Rathert, P., Zhang, X., Freund, C., Cheng, X. and Jeltsch, A. (2008) Analysis of the substrate specificity of the dim-5 histone lysine methyltransferase using Peptide arrays. Chem Biol, 15, 5-11. 135 Dhayalan, A., Kudithipudi, S., Rathert, P. and Jeltsch, A. (2011) Specificity Analysis-Based Identification of New Methylation Targets of the SET7/9 Protein Lysine Methyltransferase. Chem Biol, 18, 111-120. 136 Lu, P.J., Zhou, X.Z., Shen, M. and Lu, K.P. (1999) Function of WW domains as phosphoserine- or phosphothreonine-binding modules. Science, 283, 1325-1328. 137 Patzelt, H., Rudiger, S., Brehmer, D., Kramer, G., Vorderwulbecke, S., Schaffitzel, E., Waitz, A., Hesterkamp, T., Dong, L., Schneider-Mergener, J. et al. (2001) Binding specificity of Escherichia coli trigger factor. Proc Natl Acad Sci U S A, 98, 14244-14249. 138 Deuerling, E., Patzelt, H., Vorderwulbecke, S., Rauch, T., Kramer, G., Schaffitzel, E., Mogk, A., Schulze-Specking, A., Langen, H. and Bukau, B. (2003) Trigger Factor and DnaK possess overlapping substrate pools and binding specificities. Mol Microbiol, 47, 1317-1328. 139 Wildemann, D., Erdmann, F., Alvarez, B.H., Stoller, G., Zhou, X.Z., Fanghanel, J., Schutkowski, M., Lu, K.P. and Fischer, G. (2006) Nanomolar inhibitors of the peptidyl prolyl cis/trans isomerase Pin1 from combinatorial peptide libraries. J Med Chem, 49, 2147-2150. 140 Lee, J. and Bedford, M.T. (2002) PABP1 identified as an arginine methyltransferase substrate using high-density protein arrays. EMBO Rep, 3, 268-273. 141 Min, D.H., Yeo, W.S. and Mrksich, M. (2004) A method for connecting solution-phase enzyme activity assays with immobilized format analysis by mass spectrometry. Anal Chem, 76, 3923- 3929. 142 Schwamborn, K., Knipscheer, P., van Dijk, E., van Dijk, W.J., Sixma, T.K., Meloen, R.H. and Langedijk, J.P. (2008) SUMO assay with peptide arrays on solid support: insights into SUMO target sites. J Biochem, 144, 39-49. 143 Li, X., Vadrevu, S., Dunlop, A.J., Day, J., Advant, N., Troeger, J., Klussmann, E., Jaffray, E.G., Hay, R.T., Adams, D.R. et al. (2010) Selective SUMO modification of cAMP-specific phosphodiesterase-4D5 (PDE4D5) regulates the functional consequences of phosphorylation by PKA and ERK. Biochem J. 144 Li, X., Baillie, G.S. and Houslay, M.D. (2009) Mdm2 directs the ubiquitination of beta-arrestin- sequestered cAMP phosphodiesterase-4D5. J Biol Chem, 284, 16170-16182. 145 Laurent, N., Voglmeir, J., Wright, A., Blackburn, J., Pham, N.T., Wong, S.C., Gaskell, S.J. and Flitsch, S.L. (2008) Enzymatic glycosylation of peptide arrays on gold surfaces. Chembiochem, 9, 883-887. 146 Blixt, O., Clo, E., Nudelman, A.S., Sorensen, K.K., Clausen, T., Wandall, H.H., Livingston, P.O., Clausen, H. and Jensen, K.J. (2010) A high-throughput O-glycopeptide discovery platform for seromic profiling. J Proteome Res, 9, 5250-5261. 147 Steentoft, C., Schjoldager, K.T., Clo, E., Mandel, U., Levery, S.B., Pedersen, J.W., Jensen, K., Blixt, O. and Clausen, H. (2010) Characterization of an immunodominant cancer-specific O-glycopeptide epitope in murine podoplanin (OTS8). Glycoconj J, 27, 571-582. 148 von Olleschik-Elbheim, L., el Bayâ, A. and Schmidt, M.A. (1997) ADP-Ribosylation in Animal Tissue (Ed. Haag, Koch-Nolte), 87. 149 Clemente, S., Franco, L. and Lopez-Rodas, G. (2001) Distinct site specificity of two pea histone deacetylase complexes. Biochemistry, 40, 10671-10676. 150 Gurard-Levin, Z.A. and Mrksich, M. (2008) The activity of HDAC8 depends on local and distal sequences of its peptide substrates. Biochemistry, 47, 6242-6250. 151 Gurard-Levin, Z.A., Kim, J. and Mrksich, M. (2009) Combining mass spectrometry and peptide arrays to profile the specificities of histone deacetylases. Chembiochem, 10, 2159- 2161. 152 Fahie, K., Hu, P., Swatkoski, S., Cotter, R.J., Zhang, Y. and Wolberger, C. (2009) Side chain specificity of ADP-ribosylation by a sirtuin. FEBS J, 276, 7159-7176. 153 Smith, B.C., Settles, B., Hallows, W.C., Craven, M.W. and Denu, J.M. (2010) SIRT3 Substrate Specificity Determined by Peptide Arrays and Machine Learning. ACS Chem Biol. 154 Gurard-Levin, Z.A., Kilian, K.A., Kim, J., Bahr, K. and Mrksich, M. (2010) Peptide arrays identify isoform-selective substrates for profiling endogenous lysine deacetylase activity. ACS Chem Biol, 5, 863-873. 155 Hallows, W.C., Yu, W., Smith, B.C., Devires, M.K., Ellinger, J.J., Someya, S., Shortreed, M.R., Prolla, T., Markley, J.L., Smith, L.M. et al. (2011) Sirt3 Promotes the Urea Cycle and Fatty Acid Oxidation during Dietary Restriction. Mol Cell, 41, 139-149. 156 Duan, Y. and Laursen, R.A. (1994) Protease substrate specificity mapping using membrane- bound peptides. Anal Biochem, 216, 431-438. 157 Hilpert, K., Hansen, G., Wessner, H., Schneider-Mergener, J. and Hohne, W. (2000) Characterizing and optimizing protease/peptide inhibitor interactions, a new application for spot synthesis. J Biochem, 128, 1051-1057. 158 Dekker, N., Cox, R.C., Kramer, R.A. and Egmond, M.R. (2001) Substrate specificity of the integral membrane protease OmpT determined by spatially addressed peptide libraries. Biochemistry, 40, 1694-1701. 159 Winssinger, N., Harris, J.L., Backes, B.J. and Schultz, P.G. (2001) Angew. Chem., 113, 3254- 3258. 160 Kaup, M., Dassler, K., Reineke, U., Weise, C., Tauber, R. and Fuchs, H. (2002) Processing of the human transferrin receptor at distinct positions within the stalk region by neutrophil elastase and cathepsin G. Biol Chem, 383, 1011-1020. 161 Winssinger, N., Ficarro, S., Schultz, P.G. and Harris, J.L. (2002) Profiling protein function with small molecule microarrays. Proc Natl Acad Sci U S A, 99, 11139-11144. 162 Jones, C.H., Dexter, P., Evans, A.K., Liu, C., Hultgren, S.J. and Hruby, D.E. (2002) Escherichia coli DegP protease cleaves between paired hydrophobic residues in a natural substrate: the PapA pilin. J Bacteriol, 184, 5762-5771. 163 Salisbury, C.M., Maly, D.J. and Ellman, J.A. (2002) Peptide microarrays for the determination of protease substrate specificity. J Am Chem Soc, 124, 14868-14870. 164 Gosalia, D.N. and Diamond, S.L. (2003) Printing chemical libraries on microarrays for fluid phase nanoliter reactions. Proc Natl Acad Sci U S A, 100, 8721-8726. 165 Winssinger, N., Damoiseaux, R., Tully, D.C., Geierstanger, B.H., Burdick, K. and Harris, J.L. (2004) PNA-encoded protease substrate microarrays. Chem Biol, 11, 1351-1360. 166 Kiyonaka, S., Sada, K., Yoshimura, I., Shinkai, S., Kato, N. and Hamachi, I. (2004) Semi-wet peptide/protein array using supramolecular hydrogel. Nat Mater, 3, 58-64. 167 Janssen, S., Jakobsen, C.M., Rosen, D.M., Ricklis, R.M., Reineke, U., Christensen, S.B., Lilja, H. and Denmeade, S.R. (2004) Screening a combinatorial peptide library to develop a human glandular kallikrein 2-activated prodrug as targeted therapy for prostate cancer. Mol Cancer Ther, 3, 1439-1450. 168 Wegner, G.J., Wark, A.W., Lee, H.J., Codner, E., Saeki, T., Fang, S. and Corn, R.M. (2004) Real-time surface plasmon resonance imaging measurements for the multiplexed determination of protein adsorption/desorption kinetics and surface enzymatic reactions on peptide microarrays. Anal Chem, 76, 5677-5684. 169 Gosalia, D.N., Salisbury, C.M., Ellman, J.A. and Diamond, S.L. (2005) High throughput substrate specificity profiling of serine and cysteine proteases using solution-phase fluorogenic peptide microarrays. Mol Cell Proteomics, 4, 626-636. 170 Gosalia, D.N., Salisbury, C.M., Maly, D.J., Ellman, J.A. and Diamond, S.L. (2005) Profiling serine protease substrate specificity with solution phase fluorogenic peptide microarrays. Proteomics, 5, 1292-1298. 171 Gosalia, D.N., Denney, W.S., Salisbury, C.M., Ellman, J.A. and Diamond, S.L. (2006) Functional phenotyping of human plasma using a 361-fluorogenic substrate biosensing microarray. Biotechnol Bioeng, 94, 1099-1110. 172 Naus, S., Reipschlager, S., Wildeboer, D., Lichtenthaler, S.F., Mitterreiter, S., Guan, Z., Moss, M.L. and Bartsch, J.W. (2006) Identification of candidate substrates for ectodomain shedding by the metalloprotease-disintegrin ADAM8. Biol Chem, 387, 337-346. 173 Diaz-Mochon, J.J., Bialy, L. and Bradley, M. (2006) Dual colour, microarray-based, analysis of 10,000 protease substrates. Chem Commun (Camb), 3984-3986. 174 Han, A., Sonoda, T., Kang, J.H., Murata, M., T, N.I. and Katayam, Y. (2006) Development of a fluorescence peptide chip for the detection of caspase activity. Comb Chem High Throughput Screen, 9, 21-25. 175 Su, J., Rajapaksha, T.W., Peter, M.E. and Mrksich, M. (2006) Assays of endogenous caspase activities: a comparison of mass spectrometry and fluorescence formats. Anal Chem, 78, 4945-4951. 176 Kim, D.H., Shin, D.S. and Lee, Y.S. (2007) Spot arrays on modified glass surfaces for efficient SPOT synthesis and on-chip bioassay of peptides. J Pept Sci, 13, 625-633. 177 Inoue, Y., Mori, T., Yamanouchi, G., Han, X., Sonoda, T., Niidome, T. and Katayama, Y. (2008) Surface plasmon resonance imaging measurements of caspase reactions on peptide microarrays. Anal Biochem, 375, 147-149. 178 Kozlov, I.A., Melnyk, P.C., Hachmann, J.P., Srinivasan, A., Shults, M., Zhao, C., Musmacker, J., Nelson, N., Barker, D.L. and Lebl, M. (2008) A high-complexity, multiplexed solution-phase assay for profiling protease activity on microarrays. Comb Chem High Throughput Screen, 11, 24-35. 179 Sapsford, K.E., Sun, S., Francis, J., Sharma, S., Kostov, Y. and Rasooly, A. (2008) A fluorescence detection platform using spatial electroluminescent excitation for measuring botulinum neurotoxin A activity. Biosens Bioelectron, 24, 618-625. 180 Cedzich, A., Huttenlocher, F., Kuhn, B.M., Pfannstiel, J., Gabler, L., Stintzi, A. and Schaller, A. (2009) The protease-associated domain and C-terminal extension are required for zymogen processing, sorting within the secretory pathway, and activity of tomato subtilase 3 (SlSBT3). J Biol Chem, 284, 14068-14078. 181 Li, J. and Yao, S.Q. (2009) "Singapore Green": a new fluorescent dye for microarray and bioimaging applications. Org Lett, 11, 405-408. 182 Bovenschen, N., Quadir, R., van den Berg, A.L., Brenkman, A.B., Vandenberghe, I., Devreese, B., Joore, J. and Kummer, J.A. (2009) Granzyme K displays highly restricted substrate specificity that only partially overlaps with granzyme A. J Biol Chem, 284, 3504- 3512. 183 Collet, B.Y., Nagashima, T., Yu, M.S. and Pohl, N.L. (2009) Fluorous-based Peptide Microarrays for Protease Screening. J Fluor Chem, 130, 1042-1048. 184 Zhu, Q., Uttamchandani, M., Li, D., Lesaicherre, M.L. and Yao, S.Q. (2003) Enzymatic profiling system in a small-molecule microarray. Org Lett, 5, 1257-1260. 185 Fu, J., Cai, K., Johnston, S.A. and Woodbury, N.W. (2010) Exploring peptide space for enzyme modulators. J Am Chem Soc, 132, 6419-6424.