Student Lab Sheets Name:______
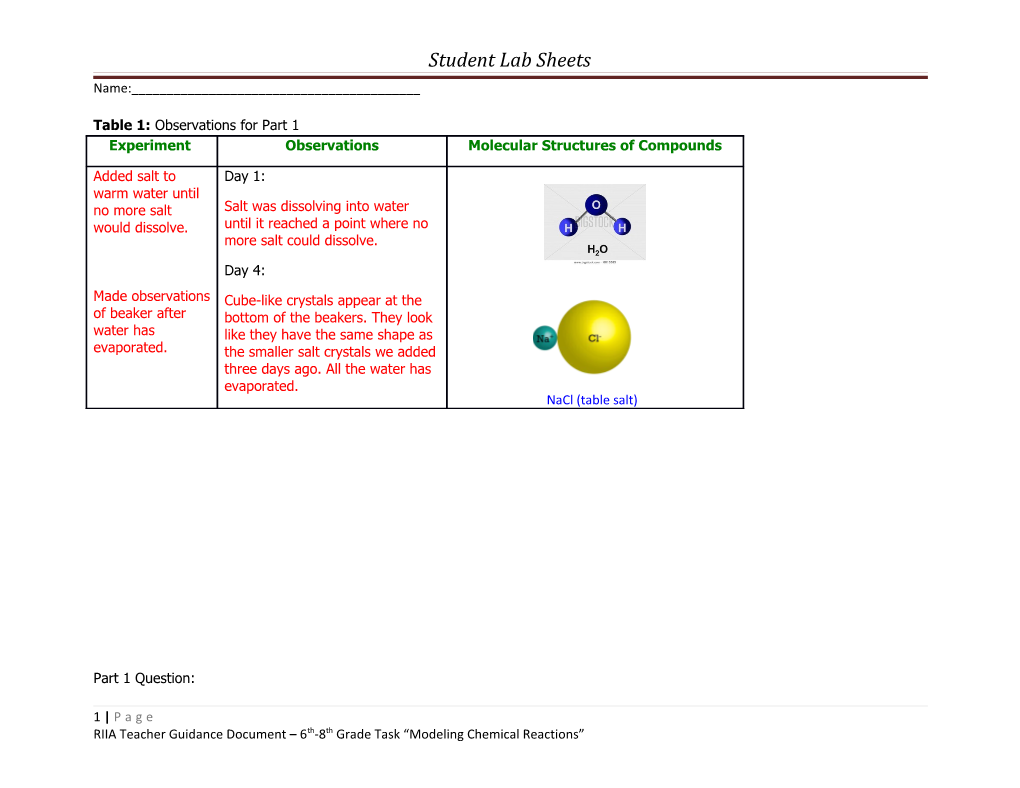
Table 1: Observations for Part 1 Experiment Observations Molecular Structures of Compounds
Added salt to Day 1: warm water until no more salt Salt was dissolving into water would dissolve. until it reached a point where no more salt could dissolve.
Day 4:
Made observations Cube-like crystals appear at the of beaker after bottom of the beakers. They look water has like they have the same shape as evaporated. the smaller salt crystals we added three days ago. All the water has evaporated. NaCl (table salt)
Part 1 Question:
1 | P a g e RIIA Teacher Guidance Document – 6th-8th Grade Task “Modeling Chemical Reactions” Student Lab Sheets Name:______How did you make models that describe the composition of salt and water molecules?
The models were made by first looking at formulas of salt and water to find out what type and how many of each type of atom we needed. In salt (NaCl) we needed one chlorine and one sodium. In water (H2O) we needed two hydrogen and one oxygen. We then selected these atoms from the molecular model kit and connected them together. After we made a 3-D model we sketched a diagram of each compound in table 1. This gave us a good idea as to the general shape and composition of salt and water.
Table 2: Sketches of Other Compounds
Compound Chemical Molecular Structure Formula
Potassium chloride KCl
Methane CH4
2 | P a g e RIIA Teacher Guidance Document – 6th-8th Grade Task “Modeling Chemical Reactions” Student Lab Sheets Name:______
Hydrogen H2
Part 2: Sketch the model of the large salt crystal in the space below.
3 | P a g e RIIA Teacher Guidance Document – 6th-8th Grade Task “Modeling Chemical Reactions” Student Lab Sheets Name:______
Part 3: Answer the following Final question:
Describe how atomic models were developed to describe the atomic composition of simple molecules and their crystalline structures?
4 | P a g e RIIA Teacher Guidance Document – 6th-8th Grade Task “Modeling Chemical Reactions” Student Lab Sheets Name:______In Parts 1 and 2, the simple models were made by first looking at formulas of each compound to find out what type and how many of each type of atom we needed. We then selected the atoms from the molecular model kit and connected them together to form 3- D models of each compound. After we made 3-D models, we sketched a diagram of each compound in table 1. From our models of simple molecules were able to see the general structure and composition of each compound.
Later in Part 2, we used a model to look at how crystallization takes place. We were able to see how crystals were formed and then construct (grow) a larger crystal structure by adding on more individual units of the molecules. That way we were able to show that lagre crystals were made up of identical smaller molecules stacked into a pattern.
5 | P a g e RIIA Teacher Guidance Document – 6th-8th Grade Task “Modeling Chemical Reactions”