Performance Benchmark P.12.A.4
Students know atoms bond with one another by transferring or sharing electrons. E/S
Atoms rarely exist as independent particles in nature. Most common substances, such as the oxygen needed to breathe, the water that makes up most of the oceans and cells, and almost every other substance imaginable are made up of combinations of atoms held together by chemical bonds. A chemical bond is a mutual attraction between the nuclei and outer electrons of different atoms that binds the atoms together.
Why are most atoms bonded to each other? Most atoms that exist as independent particles are high in potential energy when compared to atoms that are bonded. The natural tendency favors arrangements in which potential energy is minimized. For example, a ball placed on a hillside tends to roll down the hill, thus minimizing its potential energy.
Atoms are composed of three major particles: protons, neutrons, and electrons. Protons are positive particles, neutrons have no electrical charge, and electrons have a negative charge. Protons and neutrons are relatively massive, and the electron has very little mass when compared to protons and neutrons. The nucleus is where the protons and neutrons are located, and the electrons are found in the electron cloud, which occupies most of the volume of the atom.
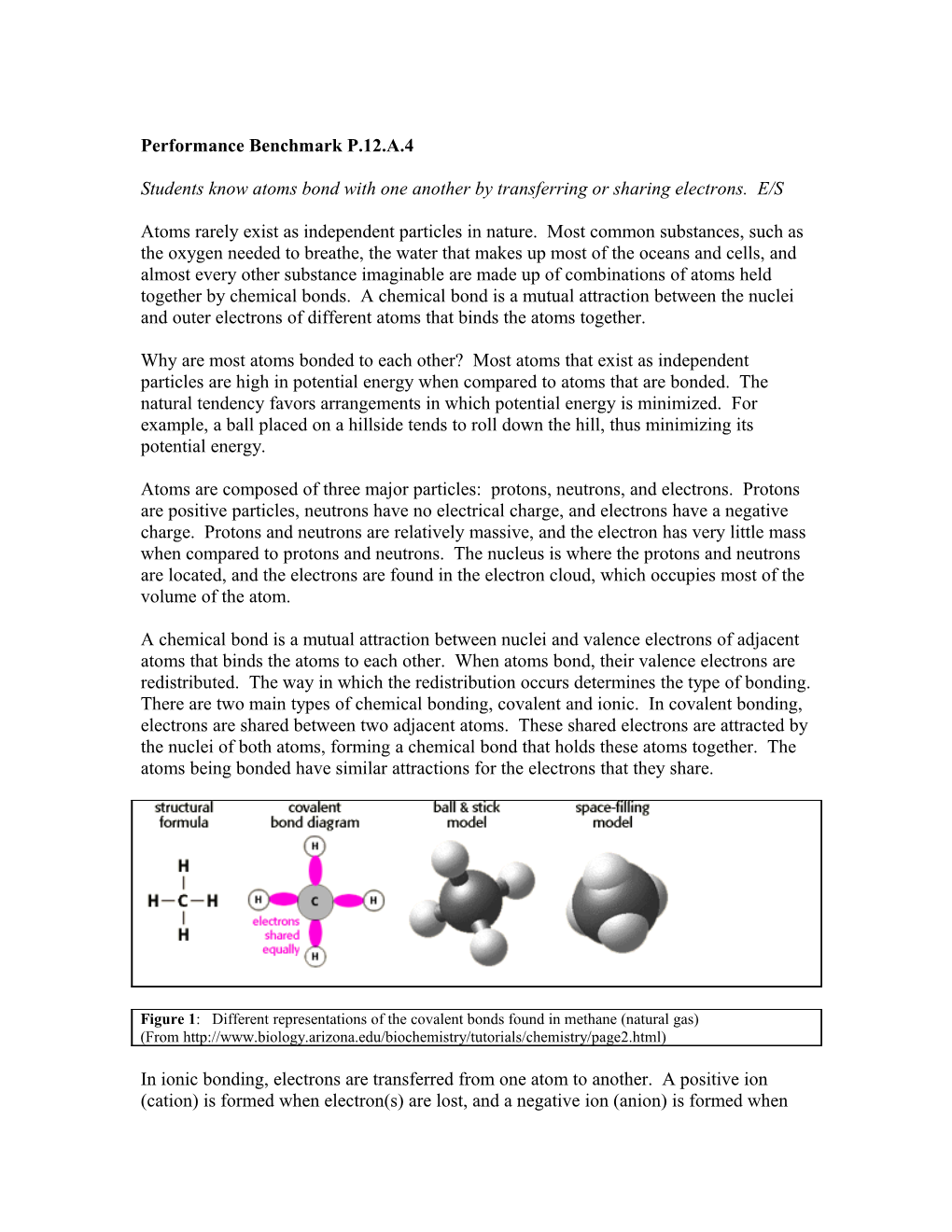
A chemical bond is a mutual attraction between nuclei and valence electrons of adjacent atoms that binds the atoms to each other. When atoms bond, their valence electrons are redistributed. The way in which the redistribution occurs determines the type of bonding. There are two main types of chemical bonding, covalent and ionic. In covalent bonding, electrons are shared between two adjacent atoms. These shared electrons are attracted by the nuclei of both atoms, forming a chemical bond that holds these atoms together. The atoms being bonded have similar attractions for the electrons that they share.
Figure 1: Different representations of the covalent bonds found in methane (natural gas) (From http://www.biology.arizona.edu/biochemistry/tutorials/chemistry/page2.html)
In ionic bonding, electrons are transferred from one atom to another. A positive ion (cation) is formed when electron(s) are lost, and a negative ion (anion) is formed when electron(s) are gained. Now that there are positive and negative ions close to each other, there is an electrostatic attraction between these oppositely charged ions, and that attraction is the ionic bond. The atoms bonded with ionic bonds have large differences in attraction for electrons so that the electrons are transferred.
Figure 2: Ionic bonding in sodium chloride (table salt) (from http://www.biology.arizona.edu/biochemistry/tutorials/chemistry/page2.html)
To learn more about bonding between atoms, go to http://web.jjay.cuny.edu/~acarpi/NSC/5-bonds.htm Performance Benchmark P.12.A.4
Students know atoms bond with one another by transferring or sharing electrons. E/S
Common misconceptions associated with this benchmark:
1. Students incorrectly think that atoms want to transfer or share electrons to form bonds.
Atoms are not alive; they do not have wants and needs. Atoms bond because it lowers their potential energy, a tendency which is favored in nature. To learn more about this, go to http://www.daisley.net/hellevator/misconceptions/misconceptions.pdf
2. Students incorrectly think that atoms are happy when they have an octet of electrons.
This is similar to misconception #1 in that students attribute feelings to explain why bonding occurs. Atoms bond because this lowers their potential energy, a natural tendency. To learn more about it, go to http://www.bcpl.net/~kdrews/bonding/bonding.html#Bond
3. Students incorrectly think that bonding requires the input of energy
Bonding always releases energy because atoms are in a lower potential energy position once the bond has been formed. To learn more about this, go to http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/bond.html Performance Benchmark P.12.A.4
Students know atoms bond with one another by transferring or sharing electrons. E/S
Sample Test Questions
1. Which particles do atoms share or transfer when they form bonds? a. protons b. neutrons c. electrons d. positrons
2. When atoms share electrons, what type of bond is formed? a. metallic b. covalent c. ionic d. hydrogen
3. When atoms transfer electrons, what type of bond is formed? a. hydrogen b. metallic c. covalent d. ionic
4. When atoms bond to each other, what type of energy change occurs? a. energy is released b. energy is absorbed c. energy is stored d. energy is unchanged
5. Why do atoms bond to one another? a. to gain an octet of electrons b. to become happy atoms c. to gain kinetic energy d. to become lower in potential energy
6. What type of attractive force is found between atoms that are bonded to one another? a. electrostatic b. magnetic c. gravitational d. nuclear 7. What happens when atoms bond to one another? a. their nuclei join together b. their electrons are transferred or shared c. their nuclei undergo fission d. their nuclei and electrons gain energy
8. In the diagram of sodium chloride above, which particles have been transferred to form the sodium and chloride ions? a. protons b. electrons c. neutrons d. nucleons
9. Above is a diagram of methane, which has the formula CH4. Which particles are shared between the carbon atom (dark central atom) and the hydrogen atoms (light atoms around carbon)? a. neutrons b. protons c. isotopes d. electrons Performance Benchmark P.12.A.4
Students know atoms bond with one another by transferring or sharing electrons. E/S
Answers to Sample Test Questions
1. (c) 2. (b) 3. (d) 4. (a) 5. (d) 6. (a) 7. (b) 8. (b) 9. (d) Performance Benchmark P.12.A.4
Students know atoms bond with one another by transferring or sharing electrons. E/S
Intervention Strategies and Resources
The following list of intervention strategies and resources will facilitate student understanding of this benchmark.
1. Chemical Bonding Flash Presentations Vision Learning has produced “Chemical Bonding”, an explanation of ionic and covalent bonding. There are flash presentations on the reaction of sodium with chlorine and of hydrogen atoms to each other. When compounds are formed, their properties are different from the properties of the elements that formed them.
The direct link to “Chemical Bonding is http://www.visionlearning.com/library/module_viewer.php?mid=55
2. Chemical Bonding Explanation “Chemical Bonding” is a short and simple explanation of covalent and ionic bonding. There are common examples (water and sodium chloride) of these types of bonding as well as of hydrogen bonding. There are some links on this page that show more detailed images of subatomic particles as well as atoms that are bonding.
To view this page, clink on the link below. http://www.nyu.edu/pages/mathmol/textbook/bonding.html
3. Covalent Bonds Explanation “Covalent Bonds” is a more complex explanation of bonding and includes other types of bonding in addition to covalent and ionic. This page also gives schematic representations (Lewis dot diagrams) of what electrons are doing in covalent and ionic bonding. Metallic bonding and hydrogen bonding are also presented.
To see this website, click on http://hyperphysics.phy- astr.gsu.edu/hbase/chemical/bond.html#c2
4. Covalent, Ionic, and Metallic Bonding Lessons “ Ithaca Science Zone” has produced lessons on covalent, ionic, and metallic bonding. Clicking on the link below will take you to covalent bonding, and there are links on this page to describe ionic and metallic bonding. Students can access only the pages they want to view. This is a very clear, concise site, and the graphics give an excellent presentation of what is happening when bonds are formed. To access the site, go to http://ithacasciencezone.com/chemzone/lessons/03bonding/mleebonding/covalent _bonds.htm