1 Topics 5 & 6 Energy and Insolation
Energy: the ability to do work or cause change. Kinetic energy: the energy of motion - the kinetic energy of the moving molecules in matter is heat or thermal energy Potential energy: energy related to position or phase (“stored energy”)
Electromagnetic Energy / Radiation
- electromagnetic energy is energy that travels in transverse waves: the waves vibrate at right angles to the direction they’re moving - the source of electromagnetic energy is the kinetic energy of moving particles of matter - the amount of thermal energy given off by an object depends on temperature……the hotter the object, the more energy it gives off - absolute zero: theoretically the lowest temperature possible (273C)……..no electromagnetic energy would be given off - radiation travels through empty space at a constant speed of 3 108 m/s (speed of light) - radiation occurs in many forms, distinguished from one another by their wavelengths
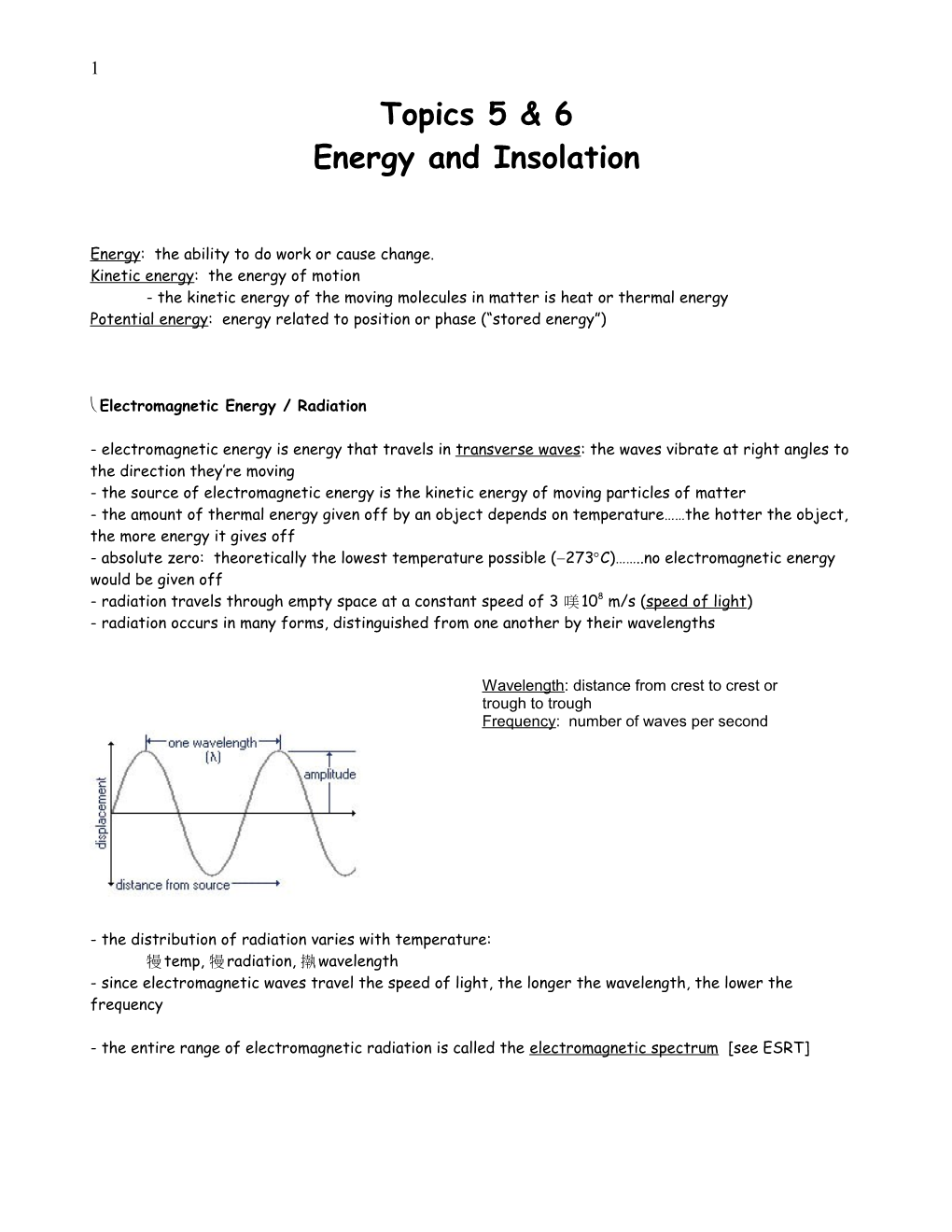
Wavelength: distance from crest to crest or trough to trough Frequency: number of waves per second
- the distribution of radiation varies with temperature: temp, radiation, wavelength - since electromagnetic waves travel the speed of light, the longer the wavelength, the lower the frequency
- the entire range of electromagnetic radiation is called the electromagnetic spectrum [see ESRT] 2
Interaction of Energy with Environment
When electromagnetic waves come into contact with matter, the waves can be: 1. refracted -- their direction is changed (“bent”) as they pass through material 2. reflected -- bounced back; waves don’t go through 3. scattered -- randomly reflected and refracted 4. absorbed -- energy is taken into material 5. transmitted -- energy passes through material without interacting
What happens to the energy depends on its wavelength and the type of material it contacts. EX/ dark, rough surfaces absorb energy best; smooth, light surfaces reflect energy best
Energy Transfer
Energy moves from place to place in three ways, based mostly on the medium through which it moves:
1. radiation -- heat energy is transferred in the form of electromagnetic waves (no molecules are involved so this can occur in empty space). EX/ starlight, microwave ovens, TV & radio waves, etc.
2. conduction -- heat energy is transferred through material by vibrating molecules Occurs best in solids because molecules are close together EX/ All metals are good conductors, allowing heat to move through easily [a material that is not a good conductor of energy is called an insulator]
3. convection -- heat energy is transferred by movement of fluids due to differences in temperature and density. Moving masses of fluids are called convection cells or convection currents. EX/ Hot air rising and cool air sinking create convection patterns as they move energy
Energy always travels from higher temperatures to lower temperatures Heat is the energy that flows from a warmer object to a cooler object (infrared radiation)
Specific Heat
- The unit of heat energy is the calorie: the amount of heat needed to raise the temperature of 1 gram of water 1 degree Celsius. - Different substances heat up at different rates - The amount of heat needed to raise the temperature of 1 gram of a substance 1C is its specific heat. - specific heat of water = 1.0 cal/g/C [see ESRT] - Water has a higher specific heat than all other naturally occurring substances; therefore, water heats up and cools down slower than equal masses of other substances. 3
Latent Heat and Phase Changes
Melting * Vaporizing Solid Liquid Gas Freezing Condensing
(sublimation)
* evaporation is when liquid becomes a gas below the boiling point
In the above diagram, moving to the right (melting, vaporizing, or evaporating) requires the absorption/addition of heat energy. Moving to the left (freezing or condensing) requires the release of heat energy.
Evaporation is said to be a cooling process because it requires heat energy to happen and it takes that energy from the surface it’s on, thereby cooling the surface down.
If you heat a beaker of water, the heat increases the kinetic energy of the molecules so the temperature of the water rises. If you continue to add heat, the temperature rises until about 100C at which point the added heat is used to break up the molecular structure of the water and change it into water vapor (steam). While the water is changing phase, the temperature doesn’t change.
Latent heat is a form of potential energy gained or lost during a phase change solid liquid = heat of fusion: 80 cal/g liquid gas = heat of vaporization: 540 cal/g
Insolation
Insolation: (INcoming SOLar radiATION) the energy from the Sun that is received on Earth 4
Intensity of Insolation depends on the angle of the Sun’s rays (90 angle = highest intensity) Factors affecting the angle of insolation: 1. Earth’s shape 2. latitude - greatest in the Tropics 3. season - greatest in summer 4. time of day - greatest at noon
Duration of Insolation: number of hours of daylight - depends on latitude and season
Temperature and Insolation Generally…….. - the greater the intensity of insolation, the higher the temperature - the greater the duration of insolation, the higher the temperature (intensity plays a greater role than duration)
In the Northern Hemisphere: - duration is greatest on June 21st - intensity is greatest on June 21st
However, June 21st is NOT the hottest day of the year…….maximum surface temperatures occur about 6 weeks later, because during this time, the Earth is radiating less heat than it is receiving and it takes time to reach a balance between terrestrial radiation (the heat the Earth is giving off) and insolation. [Likewise, the hottest time of the day is not noon, but rather around 2:00 pm]
Effects of the Atmosphere on Insolation
- Before radiation from the Sun can reach the Earth’s surface, it has to pass through the atmosphere. - Almost all of the X-rays, gamma rays, and ultraviolet (UV) rays are absorbed by ozone in Earth’s upper atmosphere and never reach Earth’s surface. These rays are all extremely dangerous to all lifeforms. [The depletion of the ozone layer due to human use of CFCs and other pollutants results in more UV rays hitting the Earth, which is increasing the amount of skin cancer that affects people] - Clouds reflect some visible light but most light passes through the atmosphere unaffected
- Most infrared radiation is absorbed by CO2 and H2O vapor in lower atmosphere - Aerosols (solid pollutants) scatter some insolation, preventing it from reaching the surface
Greenhouse Effect
Short wavelength visible light enters Earth’s atmosphere and gets absorbed by Earth’s surface. The Earth heats up and reradiates this energy as heat at longer wavelengths. This infrared radiation gets absorbed by CO2 and H2O vapor in the atmosphere, trapping the heat that would normally escape causing the temperatures to rise.
Humans add CO2 to the atmosphere by burning fossil fuels. Since plants take in CO2, human destruction of rainforests causes less CO2 to be absorbed from the atmosphere by plants. 5
Global Warming
Loss of ozone, increased amounts of CO2, and other activities of humans have led many scientists to believe that people are the cause of the worldwide increase in temperatures over the last 100 years. Other scientists argue that a 1 or 2C change in a hundred years is normal for the Earth to experience.