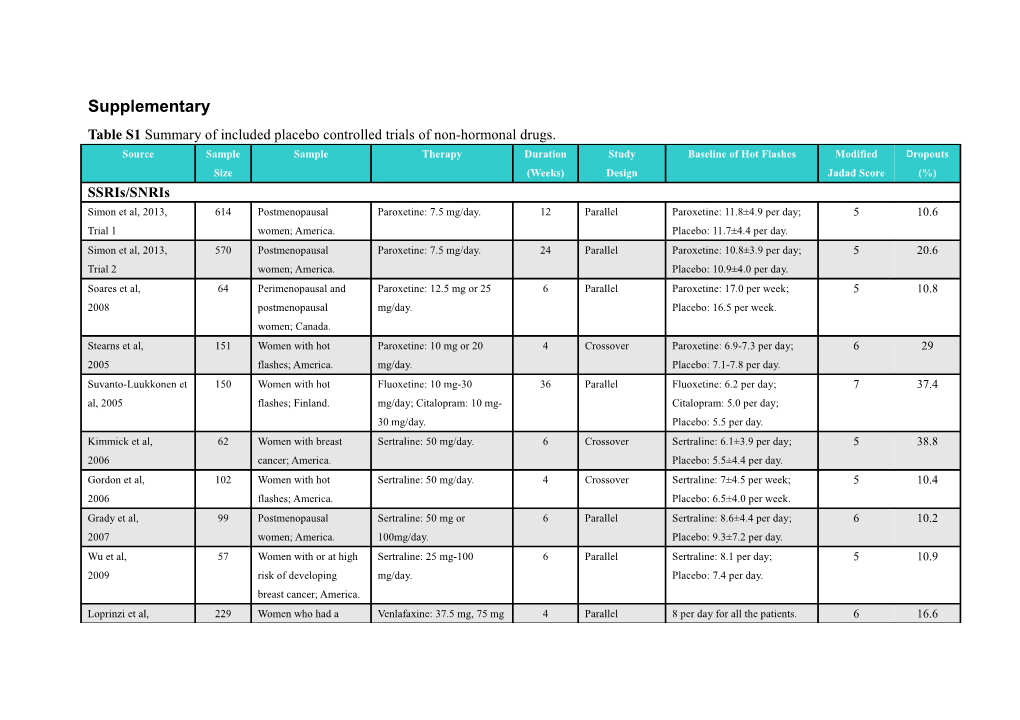
Supplementary Table S1 Summary of included placebo controlled trials of non-hormonal drugs. Source Sample Sample Therapy Duration Study Baseline of Hot Flashes Modified Dropouts Size (Weeks) Design Jadad Score (%) SSRIs/SNRIs Simon et al, 2013, 614 Postmenopausal Paroxetine: 7.5 mg/day. 12 Parallel Paroxetine: 11.8±4.9 per day; 5 10.6 Trial 1 women; America. Placebo: 11.7±4.4 per day. Simon et al, 2013, 570 Postmenopausal Paroxetine: 7.5 mg/day. 24 Parallel Paroxetine: 10.8±3.9 per day; 5 20.6 Trial 2 women; America. Placebo: 10.9±4.0 per day. Soares et al, 64 Perimenopausal and Paroxetine: 12.5 mg or 25 6 Parallel Paroxetine: 17.0 per week; 5 10.8 2008 postmenopausal mg/day. Placebo: 16.5 per week. women; Canada. Stearns et al, 151 Women with hot Paroxetine: 10 mg or 20 4 Crossover Paroxetine: 6.9-7.3 per day; 6 29 2005 flashes; America. mg/day. Placebo: 7.1-7.8 per day. Suvanto-Luukkonen et 150 Women with hot Fluoxetine: 10 mg-30 36 Parallel Fluoxetine: 6.2 per day; 7 37.4 al, 2005 flashes; Finland. mg/day; Citalopram: 10 mg- Citalopram: 5.0 per day; 30 mg/day. Placebo: 5.5 per day. Kimmick et al, 62 Women with breast Sertraline: 50 mg/day. 6 Crossover Sertraline: 6.1±3.9 per day; 5 38.8 2006 cancer; America. Placebo: 5.5±4.4 per day. Gordon et al, 102 Women with hot Sertraline: 50 mg/day. 4 Crossover Sertraline: 7±4.5 per week; 5 10.4 2006 flashes; America. Placebo: 6.5±4.0 per week. Grady et al, 99 Postmenopausal Sertraline: 50 mg or 6 Parallel Sertraline: 8.6±4.4 per day; 6 10.2 2007 women; America. 100mg/day. Placebo: 9.3±7.2 per day. Wu et al, 57 Women with or at high Sertraline: 25 mg-100 6 Parallel Sertraline: 8.1 per day; 5 10.9 2009 risk of developing mg/day. Placebo: 7.4 per day. breast cancer; America. Loprinzi et al, 229 Women who had a Venlafaxine: 37.5 mg, 75 mg 4 Parallel 8 per day for all the patients. 6 16.6 Source Sample Sample Therapy Duration Study Baseline of Hot Flashes Modified Dropouts Size (Weeks) Design Jadad Score (%) 2000 history of breast cancer; or 150 mg/day. America. Joffe et al, 339 Perimenopausal and Venlafaxine: 37.5 mg or 8 Parallel Venlafaxine: 8.2 per day; 5 6.2 2014 postmenopausal 75mg/day. Placebo: 7.7 per day. women; America. Speroff et al, 620 Postmenopausal Desvenlafaxine: 50 mg, 100 12 Parallel Desvenlafaxine: 10.5-11.2 per 7 26.6 2008 women; America. mg, 150 mg, or 200 mg/day. day; Placebo: 11.0 per day. Freedman et al, 42 Menopausal Women; Escitalopram: 10 mg or 20 8 Parallel Escitalopram: 20.6±5.2 per 7 16.2 2011 America. mg/day. day; Placebo: 20.0±5.4 per day. Freeman et al, 205 Preimenopausal or Escitalopram: 10 mg or 20 8 Parallel Escitalopram: 9.88 per day; 6 5.4 2011 postmenopausal mg/day. Placebo: 9.66 per day. women; America. Barton et al, 254 Postmenopausal Citalopram: 10mg, 20mg, or 6 Parallel Citalopram: 8-9 per day; 4 21 2010 women; America. 30 mg/day. Placebo: 8 per day. Gabapentin Butt et al, 197 Postmenopausal Gabapentin: 300 mg/day. 4 Crossover Gabapentin: 8.5 per day; 7 20.3 2008 women; Canada. Placebo: 8.5 per day. Guttuso et al, 59 Postmenopausal Gabapentin: 900 mg/day. 12 Parallel Gabapentin: 10.8±4.1 per day; 6 8.5 2003 women; American. Placebo: 10.3±3.7 per day. Pandya et al, 420 Women with breast Gabapentin: 300 mg or 900 8 Parallel Gabapentin: 8.5-8.7 per day; 6 17.4 2005 cancer; America. mg/day. Placebo: 8.8 per day. Saadati et al, 60 Postmenopausal Gabapentin: 300 mg/day. 12 Parallel Gabapentin: 13.06±4.4 per 4 not 2013 women; Iran. week; Placebo: 13.03±4.6 per reported week. Pinkerton et al, 593 Postmenopausal Gabapentin: 1800 mg/day. 24 Parallel Gabapentin: 11.8±4.7 per 4 33.8 2013 women; America. week; Placebo: 12.0±5.5 per Source Sample Sample Therapy Duration Study Baseline of Hot Flashes Modified Dropouts Size (Weeks) Design Jadad Score (%) week. Clonidine Goldberg et al, 110 Women who were Transdermalclonidine: 0.1 4 Crossover Transdermalclonidine: 6.1 per 5 19 1994 receiving tamoxifen for mg/day. day; Placebo: 7.0 per day. breast cancer; America. Laufer et al, 24 Women with hot Clonidine: 0.1 mg, 0.2 mg, 2 Parallel 0.92 per hour for all the 4 40 1982 flahses, America. or 0.4 mg/day. patients. Nagamani et al, 30 Menopausal women; Transdermalclonidine: 0.1 8 Parallel Transdermalclonidine: 41 per 4 32.1 1987 America. mg/day. week; Placebo: 36 per week. Pandya et al, 194 Postmenopausal women Clonidine: 0.1mg/day. 8 Parallel Clonidine: 8.0±0.6 per day; 6 24.7 2000 with breast cancer; Placebo: 7.4±0.5 per day. America. Soy Isoflavones Albertazzi et al, 104 Postmenopausal Soy Isoflavones: 76 mg/day. 12 Parallel Soy Isoflavones: 11.4 per day; 6 24 1998 women; Italy. Placebo: 10.9 per day. Quella et al, 177 Women who were Soy Isoflavones: 150 4 Crossover 7±4.5 per day for all the 5 15.8 2000 receiving tamoxifen for mg/day. patients. breast cancer; America. Scambia et al, 39 Menopausal women; Soy Isoflavones: 50 mg/day. 6 Parallel Soy Isoflavones: 33±5.1 per 4 not 2000 Italy week; Placebo: 27±5.1 per reported week. Upmalis et al, 177 Women with hot Soy Isoflavones: 50 mg/day. 12 Parallel Soy Isoflavones: 8.8±6.2 per 4 31.1 2000 flashes; American day; Placebo: 9.4±6.0 per day. Knight et al, 24 Postmenopausal Soy Isoflavones: 134.4 12 Parallel Soy Isoflavones: 50.2±13.6 per 6 25 2001 women; Australia. mg/day. week; Placebo: 56.2±26.5 per week. Source Sample Sample Therapy Duration Study Baseline of Hot Flashes Modified Dropouts Size (Weeks) Design Jadad Score (%) Faure et al, 75 Women with hot Soy Isoflavones: 70 mg/day. 16 Parallel Soy Isoflavones: 10.1±6.4 per 5 26.7 2002 flashes; France. day; Placebo: 9.4±3.4 per day. Van Patten et al, 157 Women with breast Soy Isoflavones: 90 mg/day. 12 Parallel Soy Isoflavones: 7.1±4.3 per 6 21.7 2002 cancer; England. day; Placebo: 7.4±6.4 per day. Penotti et al, 62 Menopausal women; Soy Isoflavones: 72 mg/day. 24 Parallel Soy Isoflavones: 9.5±3.4 per 5 21 2003 Italy. day; Placebo: 8.8±1.4 per day. Crisafulli et al, 90 Menopausal women; Soy Isoflavones: 54 mg/day. 48 Parallel Soy Isoflavones: 4.6±3.2 per 5 11.7 2004 Italy. day; Placebo: 4.7±3.2 per day. Campagnoli et al, 36 Menopausal women; Soy Isoflavones: 200 12 Crossover 38 per week for all the patients. 7 19.4 2005 Italy. mg/day. Lewis et al, 99 Menopausal women; Soy Isoflavones: 42 mg/day. 16 Parallel Soy Isoflavones: 4.1±2.4 per 6 12.1 2006 Canada. day; Placebo: 4.7±3.0 per day. Nahas et al, 80 Menopausal women; Soy Isoflavones: 100 40 Parallel Soy Isoflavones: 9.6±3.9 per 6 5.3 2007 Brazil. mg/day. day; Placebo: 10.1±4.9 per day. D'Anna et al, 236 Postmenopausal Soy Isoflavones: 54 mg/day. 96 Parallel Soy Isoflavones: 4.4±3.4 per 5 8.6 2009 women; Italy. day; Placebo: 4.2±3.7 per day. Ferrari et al, 180 Menopausal women; Soy Isoflavones: 80 mg/day. 12 Parallel Soy Isoflavones: 8.0±3.3 per 6 32.3 2009 Italy. day; Placebo: 7.5±2.8 per day. Evans et al, 84 Postmenopausal Soy Isoflavones: 30 mg/day. 12 Parallel Soy Isoflavones: 9.4±3.8 per 7 19 2011 women; Canada. day; Placebo: 9.9±3.9 per day. Ye et al, 90 Postmenopausal Soy Isoflavones: 84 mg or 24 Parallel Soy Isoflavones: 20±11.0 per 6 6.7 2012 women; China. 126 mg/day. week; Placebo: 21±12.5 per week. List of Included Articles
1. Albertazzi P, Pansini F, Bonaccorsi G, Zanotti L, Forini E, De Aloysio D (1998) The effect of dietary soy supplementation on hot flushes. Obstet Gynecol 91(1):6-11. 2. Barton DL, LaVasseur BI, Sloan JA, Stawis AN, Flynn KA, Dyar M, Johnson DB, Atherton PJ, Diekmann B, Loprinzi CL (2010) Phase III, placebo-controlled trial of three doses of citalopram for the treatment of hot flashes: NCCTG trial N05C9. J Clin Oncol 28(20):3278-3283. 3. Butt DA, Lock M, Lewis JE, Ross S, Moineddin R (2008) Gabapentin for the treatment of menopausal hot flashes: a randomized controlled trial. Menopause 15(2):310-318. 4. Campagnoli C, Abba C, Ambroggio S, Peris C, Perona M, Sanseverino P (2005) Polyunsaturated fatty acids (PUFAs) might reduce hot flushes: an indication from two controlled trials on soy isoflavones alone and with a PUFA supplement. Maturitas 51(2):127-134. 5. Crisafulli A, Marini H, Bitto A, Altavilla D, Squadrito G, Romeo A, Adamo, EB, Marini R, D'Anna R, Corrado F, Bartolone S, Frisina N, Squadrito F (2004) Effects of genistein on hot flushes in early postmenopausal women: a randomized, double-blind EPT- and placebo-controlled study. Menopause 11(4):400-404. 6. D'Anna R, Cannata ML, Marini H, Atteritano M, Cancellieri F, Corrado F, Triolo O, Rizzo P, Russo S, Gaudio A, Frisina N, Bitto A, Polito F, Minutoli L, Altavilla D, Adamo EB, Squadrito F (2009) Effects of the phytoestrogen genistein on hot flushes, endometrium, and vaginal epithelium in postmenopausal women: a 2-year randomized, double-blind, placebo-controlled study. Menopause 16(2):301-306. 7. Evans M, Elliott JG, Sharma P, Berman R, Guthrie N (2011) The effect of synthetic genistein on menopause symptom management in healthy postmenopausal women: a multi-center, randomized, placebo-controlled study. Maturitas 68(2):189-196. 8. Faure ED, Chantre P, Mares P (2002) Effects of a standardized soy extract on hot flushes:a multicenter, double-blind, randomized, placebo-controlled study. Menopause 9(5):329-334. 9. Ferrari A (2009) Soy extract phytoestrogens with high dose of isoflavones for menopausal symptoms. J Obstet Gynaecol Res 35(6):1083-1090. 10. Freedman RR, Kruger ML, Tancer ME(2011) Escitalopram treatment of menopausal hot flashes. Menopause 18(8):893-896. 11. Freeman EW, Guthrie KA, Caan B, Sternfeld B, Cohen LS, Joffe H, Carpenter JS, Anderson GL, Larson JC, Ensrud KE, Reed SD, Newton KM, Sherman S, Sammel MD, LaCroix AZ (2011) Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA 305(3):267-274. 12. Goldberg RM, Loprinzi CL, O'Fallon JR, et al. Transdermal clonidine for ameliorating tamoxifen-induced hot flashes. J Clin Oncol. 1994;12(1):155-158. 13. Gordon PR, Kerwin JP, Boesen KG, Senf J (2006) Sertraline to treat hot flashes: a randomized controlled, double-blind, crossover trial in a general population. Menopause 13(4):568-575. 14. Grady D, Cohen B, Tice J, Kristof M, Olyaie A, Sawaya GF (2007) Ineffectiveness of sertraline for treatment of menopausal hot flushes: a randomized controlled trial. Obstet Gynecol 109(4):823-30. 15. Guttuso T, Jr., Kurlan R, McDermott MP, Kieburtz K (2003) Gabapentin's effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol 101(2):337-345. 16. Joffe H, Guthrie KA, LaCroix AZ, Reed SD, Ensrud KE, Manson JE, Newton KM, Freeman EW, Anderson GL, Larson JC, Hunt J, Shifren J, Rexrode KM, Caan B. Sternfeld B, Carpenter JS, Cohen L (2014) Low-dose estradiol and the serotonin- norepinephrine reuptake inhibitor venlafaxine for vasomotor symptoms: a randomized clinical trial. JAMA Intern Med 174(7):1058-1066. 17. Kimmick GG, Lovato J, McQuellon R, Robinson E, Muss HB (2006) Randomized, double-blind, placebo-controlled, crossover study of sertraline (Zoloft) for the treatment of hot flashes in women with early stage breast cancer taking tamoxifen. Breast J 12(2):114-122. 18. Knight DC, Howes JB, Eden JA, Howes LG (2001) Effects on menopausal symptoms and acceptability of isoflavone-containing soy powder dietary supplementation.Climacteric 4(1):13-18. 19. Laufer LR, Erlik Y, Meldrum DR, Judd HL(1982) Effect of clonidine on hot flashes in postmenopausal women. Obstet Gynecol 60(5):583-586. 20. Levis S, Strickman-Stein N, Ganjei-Azar P, Xu P, Doerge DR, Krischer J (2011) Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: a randomized, double-blind trial. Arch Intern Med 171(15):1363-1369. 21. Loprinzi CL, Kugler JW, Sloan JA, Mailliard JA, LaVasseur BI, Barton DL, Novotny PJ, Dakhil SR, Rodger K, Rummans TA, Christensen BJ (2000) Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet 356(9247):2059-2063. 22. Nagamani M, Kelver ME, Smith ER (1987) Treatment of menopausal hot flashes with transdermal administration of clonidine. Am J Obstet Gynecol 156(3):561-565. 23. Nahas EA, Nahas-Neto J, Orsatti FL, Carvalho EP, Oliveira ML, Dias R (2007) Efficacy and safety of a soy isoflavone extract in postmenopausal women: a randomized, double-blind, and placebo-controlled study. Maturitas 58(3):249-258. 24. Pandya KJ, Morrow GR, Roscoe JA, ZhaoH, Hickok JT, Pajon, E, Sweeney TJ, Banerjee TK, Flynn PJ (2005) Gabapentin for hot flashes in 420 women with breast cancer: a randomised double-blind placebo-controlled trial. Lancet 366(9488):818- 824. 25. Pandya KJ, Raubertas RF, Flynn PJ, Hynes HE, Rosenbluth RJ, Kirshner JJ, Pierce HI, Dragalin V, Morrow GR (2000) Oral clonidine in postmenopausal patients with breast cancer experiencing tamoxifen-induced hot flashes: a University of Rochester Cancer Center Community Clinical Oncology Program study. Ann Intern Med 132(10):788-793. 26. Penotti M, Fabio E, Modena AB, Rinaldi M, Omodei U, Vigano P (2003) Effect of soy-derived isoflavones on hot flushes, endometrial thickness, and the pulsatility index of the uterine and cerebral arteries. Fertil Steril 79(5):1112-1117. 27. Pinkerton JV, Kagan R, Portman D, Sathyanarayana R, Sweeney M (2014) Phase 3 randomized controlled study of gastroretentive gabapentin for the treatment of moderate-to-severe hot flashes in menopause. Menopause 21(6):567-573. 28. Quella SK, Loprinzi CL, Barton DL, Knost JA, Sloan JA, LaVasseur BI, Swan D, Krupp KR, Miller KD, Novotny PJ (2000) Evaluation of soy phytoestrogens for the treatment of hot flashes in breast cancer survivors: A North Central Cancer Treatment Group Trial. J Clin Oncol 18(5):1068-1074. 29. Saadati N, Mohammadjafari R, Natanj S, Abedi P (2013) The effect of gabapentin on intensity and duration of hot flashes in postmenopausal women: a randomized controlled trial. Glob J Health Sci 5(6):126-130. 30. Scambia G, Mango D, Signorile PG, Anselmi Angeli RA, Palena C, Gallo D, Bombardelli E, Morazzoni P, Riva A, Mancuso S (2000) Clinical effects of a standardized soy extract in postmenopausal women: a pilot study. Menopause 7(2):105-111. 31. Simon JA, Portman DJ, Kaunitz AM, Mekonnen H, Kazempour K, Bhaskar S, Lippman J (2013) Low-dose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause 20(10):1027-1035. 32. Soares CN, Joffe H, Viguera AC, Petrillo L, Rydzewski M, Yehezkel R, Somley B, Cohen LS (2008) Paroxetine versus placebo for women in midlife after hormone therapy discontinuation. Am J Med 121(2):159-162. 33. Speroff L, Gass M, Constantine G, Olivier S (2008) Efficacy and tolerability of desvenlafaxine succinate treatment for menopausal vasomotor symptoms: a randomized controlled trial. Obstet Gynecol 111(1):77-87. 34. Stearns V, Slack R, Greep N, Henry-Tilman R, Osborne M, Bunnell C, Ullmer L, Gallagher A, Cullen J, Gehan E, Hayes DF, Isaacs C (2005) Paroxetine is an effective treatment for hot flashes: results from a prospective randomized clinical trial. J Clin Oncol 23(28):6919-6930. 35. Suvanto-Luukkonen E, Koivunen R, Sundstrom H, Bloigu R, Karjalainen E, Haiva-Mallinen L, Tapanainen JS (2005) Citalopram and fluoxetine in the treatment of postmenopausal symptoms: a prospective, randomized, 9-month, placebo- controlled, double-blind study. Menopause 12(1):18-26. 36. Upmalis DH, Lobo R, Bradley L, Warren M, Cone FL, Lamia CA (2000) Vasomotor symptom relief by soy isoflavone extract tablets in postmenopausal women: a multicenter, double-blind, randomized, placebo-controlled study. Menopause 7(4):236-242. 37. Van Patten CL, Olivotto IA, Chambers GK, Gelmon KA, Hislop TG, Templeton E, Wattie A, Prio JC (2002) Effect of soy phytoestrogens on hot flashes in postmenopausal women with breast cancer: a randomized, controlled clinical trial. J Clin Oncol 20(6):1449-1455. 38. Wu MF, Hilsenbeck SG, Tham YL, Kramer R, Elledge RM, Chang JC, Friedman LC (2009) The efficacy of sertraline for controlling hot flashes in women with or at high risk of developing breast cancer. Breast Cancer Res Treat 118(2):369-375. 39. Ye YB, Wang ZL, Zhuo SY, Lu W, Liao HF, Verbruggen M, Fang S, Mai HY, Chen YM, Su YX (2012) Soy germ isoflavones improve menopausal symptoms but have no effect on blood lipids in early postmenopausal Chinese women:a randomized placebo-controlled trial. Menopause 19(7):791-798.
Figure S1 Goodness-of-fit plots for the model. A) Population predicted effect data vs. observed effect data; B) individual predicted effect data vs. observed effect data; C) weighted residuals vs. population predicted effect data; D) density distribution of weighted residuals. The black and red lines in A and B represent identity and regression lines, respectively, whereas in C the black line is the position where weighted residual equals 0 and the red lines are the nonparametric regression lines. The red line in D is density distribution line.
SSRIs/SNRIs Gabapentin Clonidine Soy Isoflavones A
B
C
D
Figure S2 The distribution of typical parameters obtained from the model in a Jackknife method.