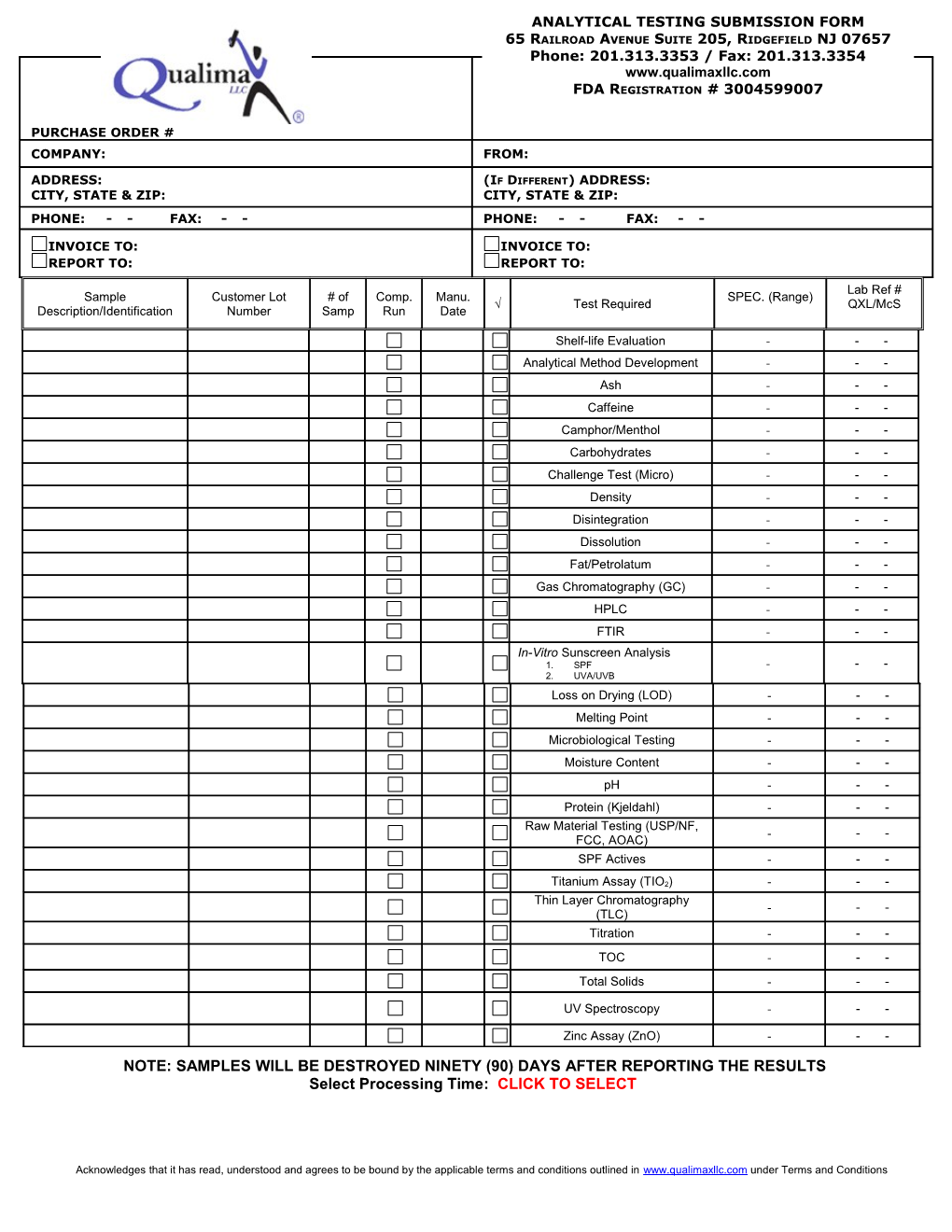
ANALYTICAL TESTING SUBMISSION FORM 65 RAILROAD AVENUE SUITE 205, RIDGEFIELD NJ 07657 Phone: 201.313.3353 / Fax: 201.313.3354 www.qualimaxllc.com FDA REGISTRATION # 3004599007 SUBMISSION DATE:
PURCHASE ORDER # COMPANY: FROM:
ADDRESS: (IF DIFFERENT) ADDRESS: CITY, STATE & ZIP: CITY, STATE & ZIP: PHONE: - - FAX: - - PHONE: - - FAX: - -
INVOICE TO: INVOICE TO: REPORT TO: REPORT TO: Lab Ref # Sample Customer Lot # of Comp. Manu. SPEC. (Range) √ Test Required QXL/McS Description/Identification Number Samp Run Date
Shelf-life Evaluation - - - Analytical Method Development - - - Ash - - - Caffeine - - - Camphor/Menthol - - - Carbohydrates - - - Challenge Test (Micro) - - - Density - - - Disintegration - - - Dissolution - - - Fat/Petrolatum - - - Gas Chromatography (GC) - - - HPLC - - - FTIR - - - In-Vitro Sunscreen Analysis 1. SPF - - - 2. UVA/UVB Loss on Drying (LOD) - - - Melting Point - - - Microbiological Testing - - - Moisture Content - - - pH - - - Protein (Kjeldahl) - - - Raw Material Testing (USP/NF, - - - FCC, AOAC) SPF Actives - - -
Titanium Assay (TIO2) - - - Thin Layer Chromatography - - - (TLC) Titration - - - TOC - - - Total Solids - - -
UV Spectroscopy - - -
Zinc Assay (ZnO) - - - NOTE: SAMPLES WILL BE DESTROYED NINETY (90) DAYS AFTER REPORTING THE RESULTS Select Processing Time: CLICK TO SELECT
Acknowledges that it has read, understood and agrees to be bound by the applicable terms and conditions outlined in www.qualimaxllc.com under Terms and Conditions