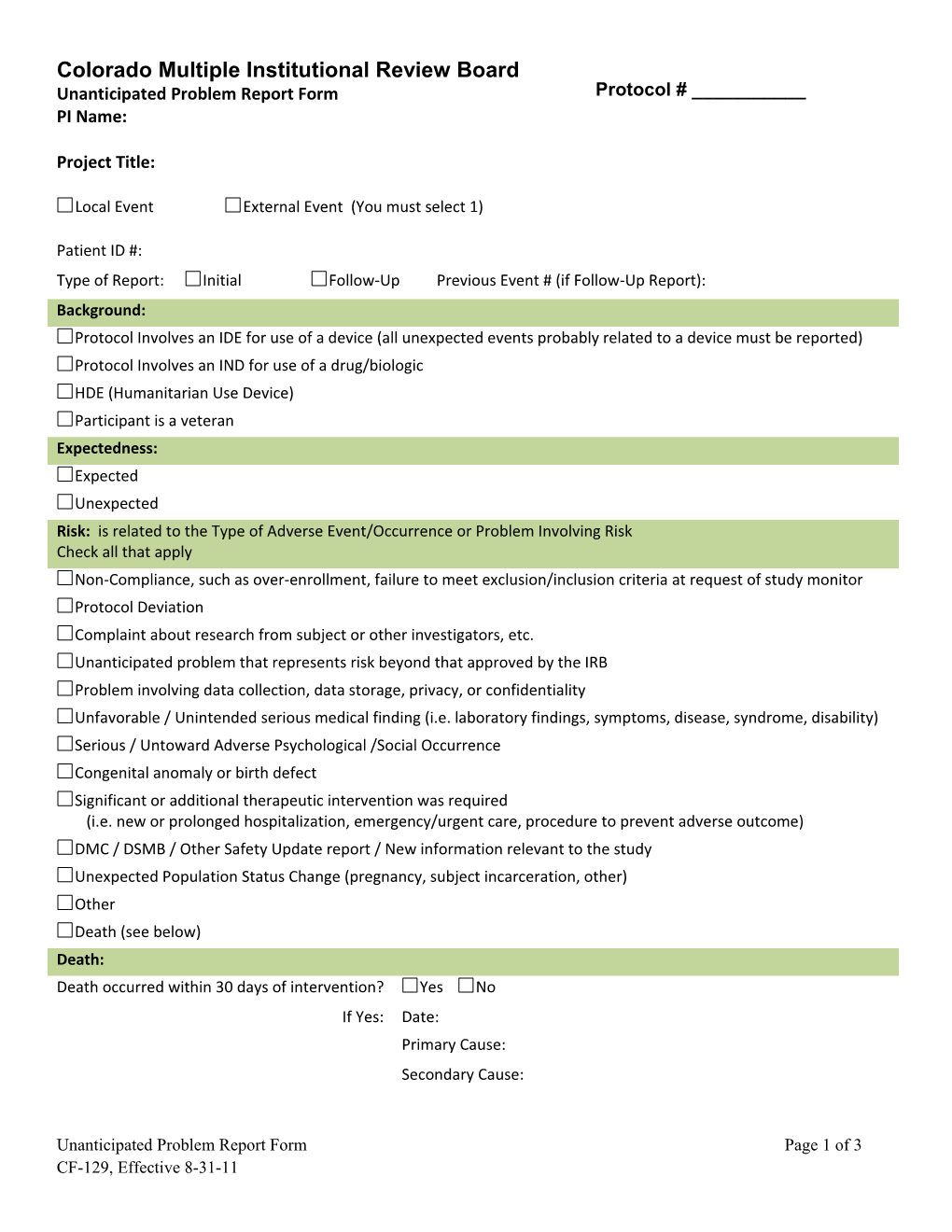
Colorado Multiple Institutional Review Board Unanticipated Problem Report Form Protocol # ______PI Name:
Project Title:
Local Event External Event (You must select 1)
Patient ID #: Type of Report: Initial Follow-Up Previous Event # (if Follow-Up Report): Background: Protocol Involves an IDE for use of a device (all unexpected events probably related to a device must be reported) Protocol Involves an IND for use of a drug/biologic HDE (Humanitarian Use Device) Participant is a veteran Expectedness: Expected Unexpected Risk: is related to the Type of Adverse Event/Occurrence or Problem Involving Risk Check all that apply Non-Compliance, such as over-enrollment, failure to meet exclusion/inclusion criteria at request of study monitor Protocol Deviation Complaint about research from subject or other investigators, etc. Unanticipated problem that represents risk beyond that approved by the IRB Problem involving data collection, data storage, privacy, or confidentiality Unfavorable / Unintended serious medical finding (i.e. laboratory findings, symptoms, disease, syndrome, disability) Serious / Untoward Adverse Psychological /Social Occurrence Congenital anomaly or birth defect Significant or additional therapeutic intervention was required (i.e. new or prolonged hospitalization, emergency/urgent care, procedure to prevent adverse outcome) DMC / DSMB / Other Safety Update report / New information relevant to the study Unexpected Population Status Change (pregnancy, subject incarceration, other) Other Death (see below) Death: Death occurred within 30 days of intervention? Yes No If Yes: Date: Primary Cause: Secondary Cause:
Unanticipated Problem Report Form Page 1 of 3 CF-129, Effective 8-31-11 Relationship of Unanticipated Problem to Study: Not all adverse events may involve treatement (e.g. risk involving confidentiality or psychological outcomes) Select the ONE that best applies Not Related Unlikely Related / Possibly Reasonable Probability it is Related / Probably Definitely Related Unknown Not Applicable
Previous Reporting of similar Unanticipated Problems: Has this type of event been Yes No If yes, list the # of Times Local: reported previously to COMIRB? If yes, list the # of Times Off Site: Is this event named in the Yes No consent form as a risk? If yes, Page #: If No, Should it be named in the consent? Yes No If Yes, submit changes on Alteration/Update Form If No above, please Justify not including in the consent:
Do you recommend Protocol Yes No If Yes, submit a Protocol Amendment Form Changes? Accrual Information: Total Number of LOCAL subjects Approved by COMIRB? Current LOCAL Accrual? Anticipated Target Accrual across all sites? (Should be in the Protocol) This is a Multi-Institutional Study Timeline: Date Event Started? Date Event Ended/Resolved? Event is ongoing (not resolved) Date local PI/coordinator became aware of event?
Unanticipated Problem Report Form Page 2 of 3 CF-129, Effective 8-31-11 Describe the Problem: (Include Dates and Sequence of Events)
Unanticipated Problems must be submitted within five (5) days of when the event started.
Type name of PI (or persons filing report*):
Signature: Date:
* PI is required to sign the Unanticipated Problem report. If PI will not be available within the 5 day mandatory reporting period, a Co-Investigator may file this report for the PI
Unanticipated Problem Report Form Page 3 of 3 CF-129, Effective 8-31-11