4.2 Representing Molecular Compounds (Page 152) Name:______
______are usually composed of two or more different ______. The elements within a molecular compound ______.
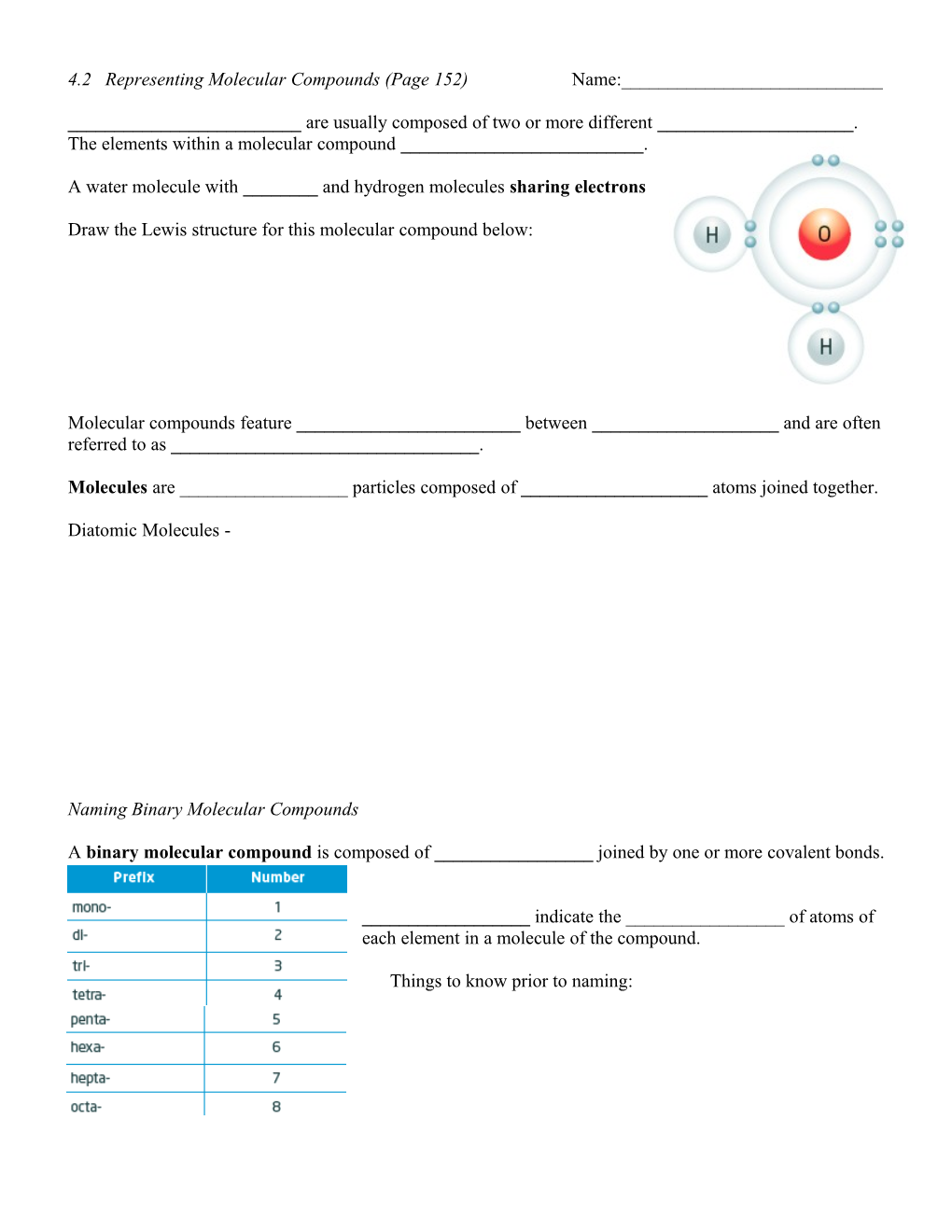
A water molecule with ______and hydrogen molecules sharing electrons
Draw the Lewis structure for this molecular compound below:
Molecular compounds feature ______between ______and are often referred to as ______.
Molecules are ______particles composed of ______atoms joined together.
Diatomic Molecules -
Naming Binary Molecular Compounds
A binary molecular compound is composed of ______joined by one or more covalent bonds.
______indicate the ______of atoms of each element in a molecule of the compound.
Things to know prior to naming: Step 1: Use prefixes to indicate the number of both atoms in the compound (given in the chemical formula)
Step 2: The name of the second element ends in –ide. *Write the elements in order of how the appear in the chemical formula*
Example 1: Name SO3 Example 2: Name N2O4
Writing Chemical Formulas for Molecular Compounds Example: Write the chemical formula for disulfur dinitride
Step 1: Write down the symbol of the elements with the subscripts indicated by the prefixes.
Note:
(A simplified ratio can result in a different molecular compound. For example, NO2 and N2O4 are different compounds!)
Common names of molecular compounds
Practice: Name each of the following compounds. a) BrF ______f) PBr5 ______b) NO ______g) Br2O ______c) CO2 ______h) P2O3______d) SO3 ______i) CO ______e) As2O3 ______j) P4O10 ______
Write the chemical formula for each of the following compounds. a) Iodine monofluoride ______f) carbon tetrafluoroide ______b) Arsenic trichloride ______g) hydrogen chloride ______c) sulfur dioxide ______h) dichlorine monoxide ______d) phosphorus pentabromide ______i) dichlorine pentoxide ______e) nitrogen trichloride ______j) sulfur hexafluoride ______
Homework Read Pages 152-158 and Answer Q’s 1-3, 6 on Page 158 Read Pages 159-168 and Answer Q’s 1, 3, 5, 6 on Page 168