Lab: Synthesis of Esters
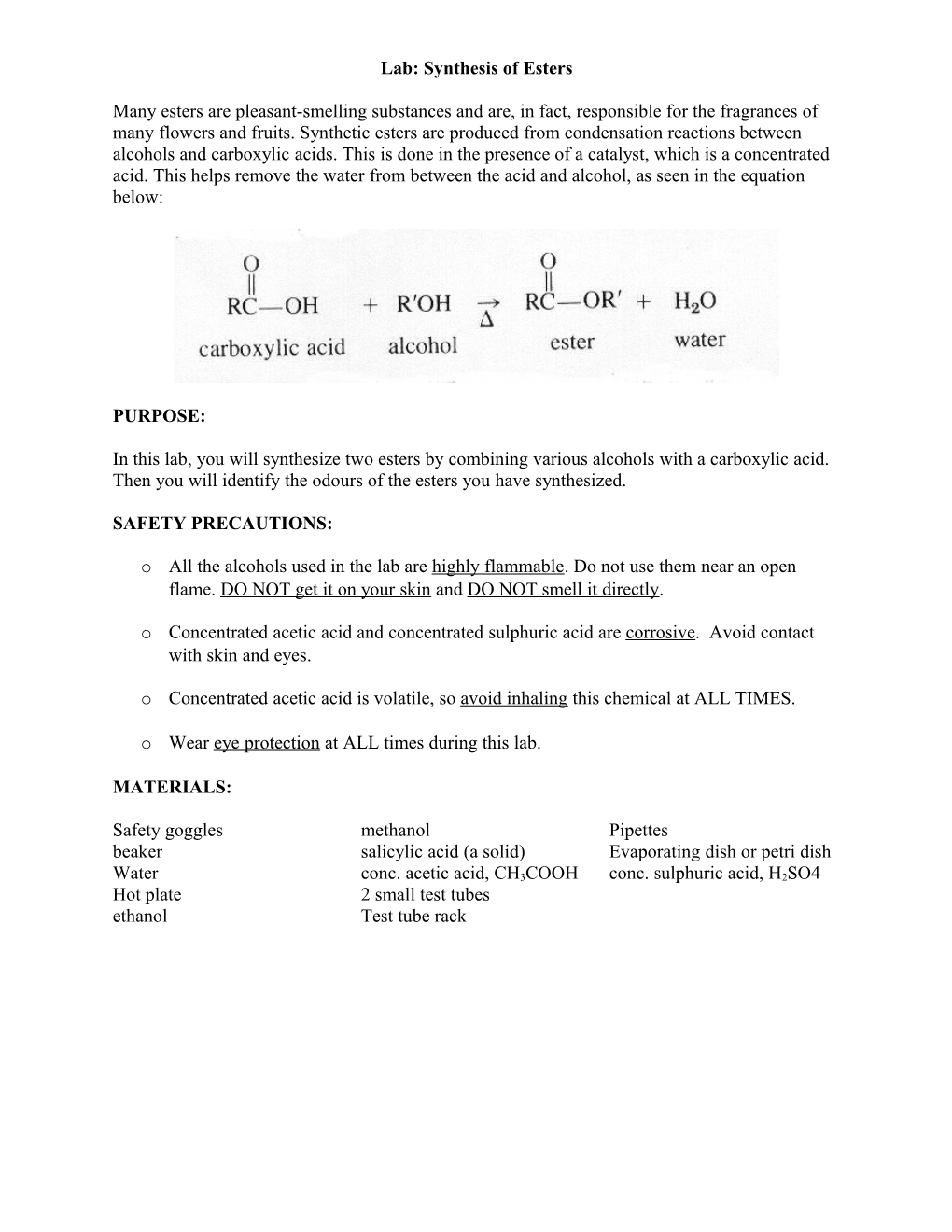
Many esters are pleasant-smelling substances and are, in fact, responsible for the fragrances of many flowers and fruits. Synthetic esters are produced from condensation reactions between alcohols and carboxylic acids. This is done in the presence of a catalyst, which is a concentrated acid. This helps remove the water from between the acid and alcohol, as seen in the equation below:
PURPOSE:
In this lab, you will synthesize two esters by combining various alcohols with a carboxylic acid. Then you will identify the odours of the esters you have synthesized.
SAFETY PRECAUTIONS:
o All the alcohols used in the lab are highly flammable. Do not use them near an open flame. DO NOT get it on your skin and DO NOT smell it directly.
o Concentrated acetic acid and concentrated sulphuric acid are corrosive. Avoid contact with skin and eyes.
o Concentrated acetic acid is volatile, so avoid inhaling this chemical at ALL TIMES.
o Wear eye protection at ALL times during this lab.
MATERIALS:
Safety goggles methanol Pipettes beaker salicylic acid (a solid) Evaporating dish or petri dish Water conc. acetic acid, CH3COOH conc. sulphuric acid, H2SO4 Hot plate 2 small test tubes ethanol Test tube rack PROCEDURE:
NOTE: READ THE ENTIRE SET OF INSTRUCTION BEFORE STARTING THE LAB!
1. Fill up HALF of the beaker with water. Heat the beaker carefully on a hot plate until the water comes to a gentle boil. Maintain the temperature at 60-65°C. Do NOT let it go over this temperature, so adjust the temperature accordingly. A thermometer will help you maintain the temperature.
2. Two test tubes have been labelled for you (#1, #2). The chart below tells you what you should add. READ STEPS 3 AND 4 CAREFULLY BEFORE PROCEEDING.
Contents Test Tube #1 Test Tube #2 10 drops 15 drops Alcohol ethanol methanol 10 drops 1 small scoop Acid conc. acetic acid salicylic acid (0.5 g) 2 drops 2 drops Catalyst conc. sulphuric acid conc. sulphuric acid
3. In the fume hood, use the pipette to add 10 drop of ethanol to the test tube. THEN, very carefully, add 10 drops of concentrated acetic acid to test tube # 1. THEN add 2 DROPS of concentrated sulphuric acid VERY CAREFULLY. Gentle shake the test tube to mix its contents. DO NOT SMELL ANYTHING DIRECTLY.
4. In the fume hood, scoop A SMALL amount of salicylic acid and add it to test tube #2. Add 15 drops of methanol very carefully. THEN add 2 DROPS of concentrated sulphuric acid VERY CAREFULLY. Gentle shake the test tube to mix its contents. DO NOT SMELL ANYTHING DIRECTLY.
5. Carefully return the test tubes to your station and place the test tubes in the water bath. Be careful to not point the test tubes at anybody.
6. After 5 minutes of heating (test tubes have been in a constant 60-65°C water bath for 5 minutes), remove the test tubes from the heat using tongs and place them carefully on the test tube rack. Let the test tubes cool to room temperature.
7. You have evaporating dishes (or glass petri dishes). Fill up each dish to the half-way mark with COLD DISTILLED WATER.
8. Pour the contents of the 1st test tube into an evaporating dish (or a glass petri dish). Identify the odour of the ester by gently WAFTING the scent towards your nose. NEVER INHALE DIRECTLY. Do the same thing with the 2nd test tube in the other evaporating dish.
9. Dispose the contents of the dishes into the organic waste beaker, as directed by your teacher for further disposal.
10. Wash all the glassware from your lab extremely well and hang on the drying rack.
11. Clean up your lab station, put away the equipment and wash your hands extremely well. Questions – answer on separate sheet of paper, answering in FULL sentences.
1. Fill in the table below. Reaction 1 Reaction 2 (Test Tube 1) (Test Tube 2) IUPAC name of alcohol
Structural formula of alcohol
IUPAC name of carboxylic acid
Structural formula of carboxylic acid
IUPAC name of ester produced
Structural formula of ester produced
Odour of ester produced
2. What was the function of the concentrated sulphuric acid in these reactions? 3. Why were you instructed to use an electric hot plate instead of a Bunsen burner in this lab? 4. Why did you use warm water instead of hot boiling water? 5. Write a structural formula equation showing one of the two esterifications in this lab below. 6. Give three commercial uses for synthetic esters 7. Explain why there are differences between artificial flavourings and natural flavourings.