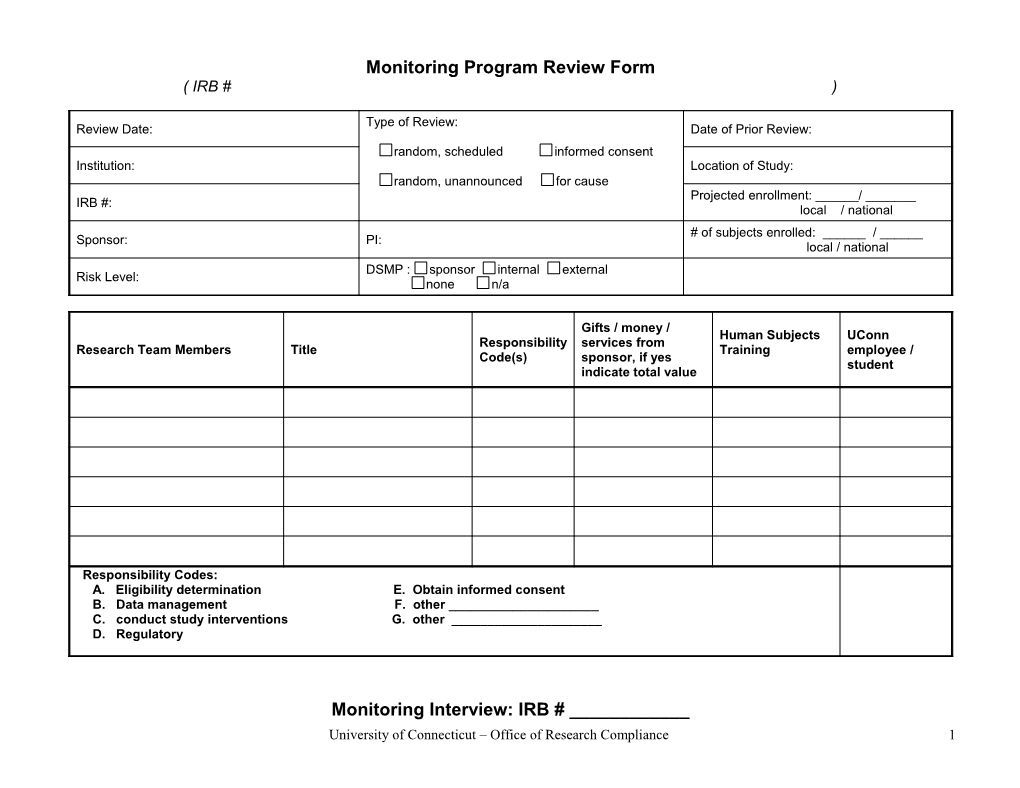
Monitoring Program Review Form ( IRB # )
Type of Review: Review Date: Date of Prior Review: random, scheduled informed consent Institution: Location of Study: random, unannounced for cause Projected enrollment: ______/ ______IRB #: local / national # of subjects enrolled: ______/ ______Sponsor: PI: local / national DSMP : sponsor internal external Risk Level: none n/a
Gifts / money / Human Subjects UConn Responsibility services from Research Team Members Title Training employee / Code(s) sponsor, if yes student indicate total value
Responsibility Codes: A. Eligibility determination E. Obtain informed consent B. Data management F. other ______C. conduct study interventions G. other ______D. Regulatory
Monitoring Interview: IRB # ______University of Connecticut – Office of Research Compliance 1 Question Answer
Date study received final approval? Date of 1st recruitment activity and by what method (eg ad posted, radio announcement, etc.) Describe recruitment methods used in this study
) Are all recruitment methods IRB approved? E C I
T Describe the diversity of subjects recruited; gender, race, age, etc. S
U Describe storage and security of subject files/ electronic data? Where are they J (
kept, who has access, what security measures? T Is consent obtained before screening procedures? N
E Is private identifiable information collected? Waiver advisable? M T
I How much time is spent on consent process in the office/clinic? U
R Do subjects have time to review consent at home, with friends/family? C E
R Do subjects receive a copy of the signed consent?
When do subjects receive payments / reimbursements? Per visit? At end?
Is this advertised on web site?
Vulnerable Populations Recruitment totals
How many recruits are a relative or friend of the research team? How many subjects screened?
How many recruits are UConn employees or students? How many subjects consented? How many recruits are a child, prisoner, pregnant woman, neonate or How many screened failures? unborn? How many recruits are decisionally-impaired? How many screened withdrawals? Please describe. How many subjects enrolled in study?
Informed Consent Content Review: IRB # ______
University of Connecticut – Office of Research Compliance 2 Required Elements 45 CFR 46.116(a)
ICF valid Involves Risks & Reasonable Alternatives Confidentiality Compensation Contacts / Voluntary date research discomforts benefits & injury rights 46.116.(a)(1) 46.116.(a)(2) 46.116.(a)(3) 46.116.(a)(4) 46.116/(a)(5) 46.116(a)(6) 46.116(a)(7) 46.116(a)(8)
Additional Elements 45 CFR 46.116(b)
ICF valid Termination without Consequences of voluntary New findings which may Language date subject consent withdrawal influence participation 45CFR46.116 46.116(b)(2) 46.116(b)(2) 46.116(b)(5) Complexity Exculpatory
Informed Consent Content Assessment
Acceptable Acceptable, needs follow-up Unacceptable
Informed Consent Process Review: IRB # ______
Consent Item Subject ID# Subject ID# Subject ID# Subject ID# Subject ID# University of Connecticut – Office of Research Compliance 3 record ID # in shaded area Last name of person obtaining consent Consenter is qualified/delegated by PI PI involved in consent process Approximate time spent on consenting process Consent is written /explained in primary language of the subject Subject signed / dated ICF Subject received a copy of ICF Subject needed assistance in language or other (describe) Subject meets eligibility requirements Date of 1st study-related procedure / test / event / or screening Date stamped, valid ICF signed ICF witnessed (if applicable) Research record is stored securely Data is labeled according to ICF
CONSENT: SUBJECT INTERVIEW (if applicable) Subject understands study purpose Subject understands risks Subject understands benefits (personal or to society) Subject understand reasonable alternatives to participation Subject understands participation is voluntary Subject understands costs / compensation Subject understands time commitment for participation Subject understands withdrawal and/or termination from study Subject understands confidentiality Subject is aware of contacts regarding questions or injuries
Consent Compliance Assessment
Acceptable Acceptable, needs follow-up Unacceptable
Participant File Review: IRB # ______
Subject ID# Subject ID# Subject ID# Subject ID# Subject ID# Participant Review Item University of Connecticut – Office of Research Compliance 4 Informed Consent (stamped and valid version) E C
N Eligibility (verification) E R E H D A Study Interventions L O C O T
O Study Calendar / Visit R
P schedule
Data quality, source documents
Data /chart security
Labeling & identifiers
Payments / Compensation
Case Review Compliance Assessment
Acceptable Acceptable, needs follow-up Unacceptable
Regulatory Compliance: IRB # ______
University of Connecticut – Office of Research Compliance 5 Approval Type of Review Full / Review Consent IRB Minutes Protocol Recorded in: Comments Date Expedited materials changes Initial / Cont / Mod Exempt Quorum, COI, adequacy Version Yes / No IRB Study Deficiencies / other diversity
Regulatory Compliance Assessment
Acceptable Acceptable, needs follow-up Unacceptable
Monitoring Summary: IRB # ______
University of Connecticut – Office of Research Compliance 6 Overview
Acceptable Acceptable, needs follow-up Unacceptable
Comments:
Description of any deficiencies / violations:
Recommendations:
SIGNATURES
Role Printed Name Title Signature Date
Prepared Report NAME IRB Monitor
Approved Report NAME Director, ORC
Approved Report NAME IRB Chair/Co-Chair
University of Connecticut – Office of Research Compliance 7