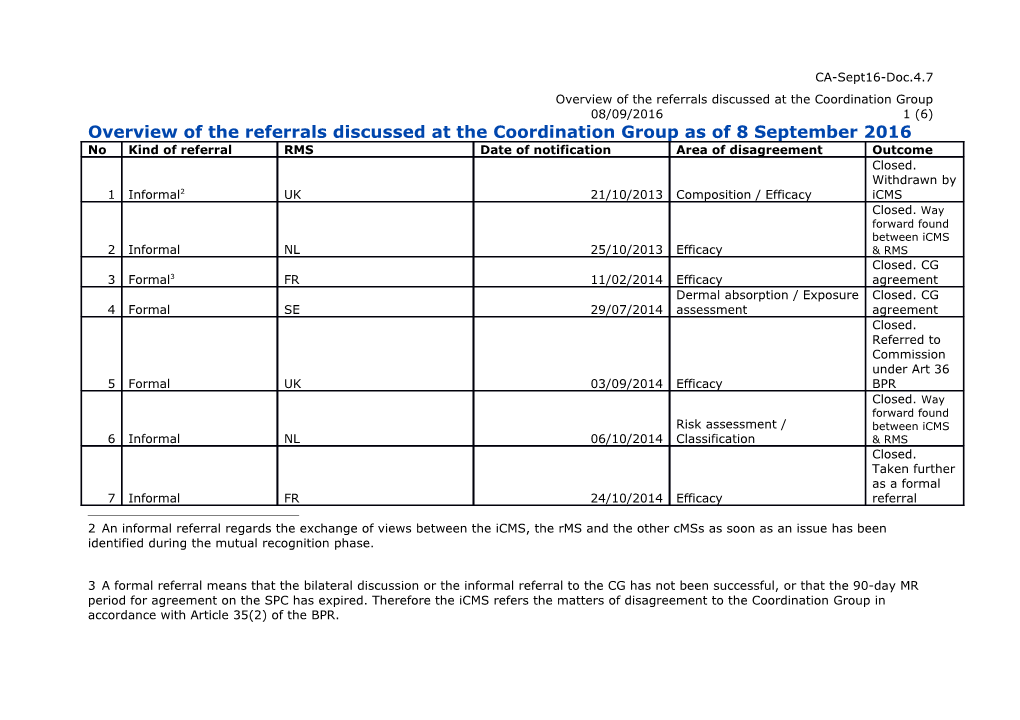
CA-Sept16-Doc.4.7 Overview of the referrals discussed at the Coordination Group 08/09/2016 1 (6) Overview of the referrals discussed at the Coordination Group as of 8 September 2016 No Kind of referral RMS Date of notification Area of disagreement Outcome Closed. Withdrawn by 1 Informal2 UK 21/10/2013 Composition / Efficacy iCMS Closed. Way forward found between iCMS 2 Informal NL 25/10/2013 Efficacy & RMS Closed. CG 3 Formal3 FR 11/02/2014 Efficacy agreement Dermal absorption / Exposure Closed. CG 4 Formal SE 29/07/2014 assessment agreement Closed. Referred to Commission under Art 36 5 Formal UK 03/09/2014 Efficacy BPR Closed. Way forward found Risk assessment / between iCMS 6 Informal NL 06/10/2014 Classification & RMS Closed. Taken further as a formal 7 Informal FR 24/10/2014 Efficacy referral
2 An informal referral regards the exchange of views between the iCMS, the rMS and the other cMSs as soon as an issue has been identified during the mutual recognition phase.
3 A formal referral means that the bilateral discussion or the informal referral to the CG has not been successful, or that the 90-day MR period for agreement on the SPC has expired. Therefore the iCMS refers the matters of disagreement to the Coordination Group in accordance with Article 35(2) of the BPR. No Kind of referral RMS Date of notification Area of disagreement Outcome Closed. Referred to Commission Efficacy / Exposure under Art 36 8 Formal ES 17/12/2014 assessment BPR Closed. Way forward found between 9 Informal DE 13/01/2015 Composition iCMS & RMS Closed. CG 10 Formal FR 19/01/2015 Efficacy agreement Closed. To be taken further as a formal 11 Informal DE 16/02/2015 Risk assessment referral Closed. Withdrawal of Risk assessment / authorisation 12 Formal NL 03/03/2015 Classification s
No Kind of referral iCMS4 RMS PT Date of notification CG meeting discussion Area of disagreement Outcome 13 Formal
4 iCMS: Initiating concerned MS that starts the referral process under a mutual recognition procedure. CA-Sept16-Doc.4.7 Overview of the referrals discussed at the Coordination Group 08/09/2016 3 (6) DE UK 18 20/04/2015 CG-11&12 Composition / Risk assessment Closed. CG agreement 14 Formal DE UK 8 11/05/2015 CG-11&12 Identified SoCs / Risk assessment Closed. CG agreement 15 Formal DK DE 1 22/06/2015 CG-12&13 Risk assessment Closed. CG agreement 16 Formal DE IT 14 23/09/2015 CG-14 Efficacy Closed. CG agreement 17 Formal DE UK 18 26/10/2015 CG-14, 15 Environmental risk assessment Closed. Referred to Commission under Art 36 BPR 18 Formal FR UK 8 18/01/2016 CG-16 Phys-chem properties, efficacy and human health risk assessment Closed. CG agreement 19 Formal FR DE 8 18/01/2016 CG-16 Human health risk assessment Closed. CG agreement 20 Formal DE UK 8 22/01/2016 CG-16 Efficacy Closed. CG agreement CA-Sept16-Doc.4.7 Overview of the referrals discussed at the Coordination Group 08/09/2016 5 (6) 21 Formal DE DK 8 29/01/2016 CG-16 Human health risk assessment Closed. CG agreement 22 Formal DE FR 18 14/04/2016 CG-17 Human health risk assessment Closed. CG agreement 23 Formal NL LV 1,2 and 4 11/05/2016 CG- 17 & CG-18 Efficacy Closed. CG agreement 24 Formal FR UK 18 23/05/2016 CG-18 Efficacy Closed. CG agreement 25 Formal DE IT 14 08/06/2016 CG-18 RMM Closed. CG agreement 26 Formal FR DE 18 22/08/2016 CG-19 Efficacy Under discussion 27 Formal FR DE 18 22/08/2016 CG-19 Efficacy Under discussion