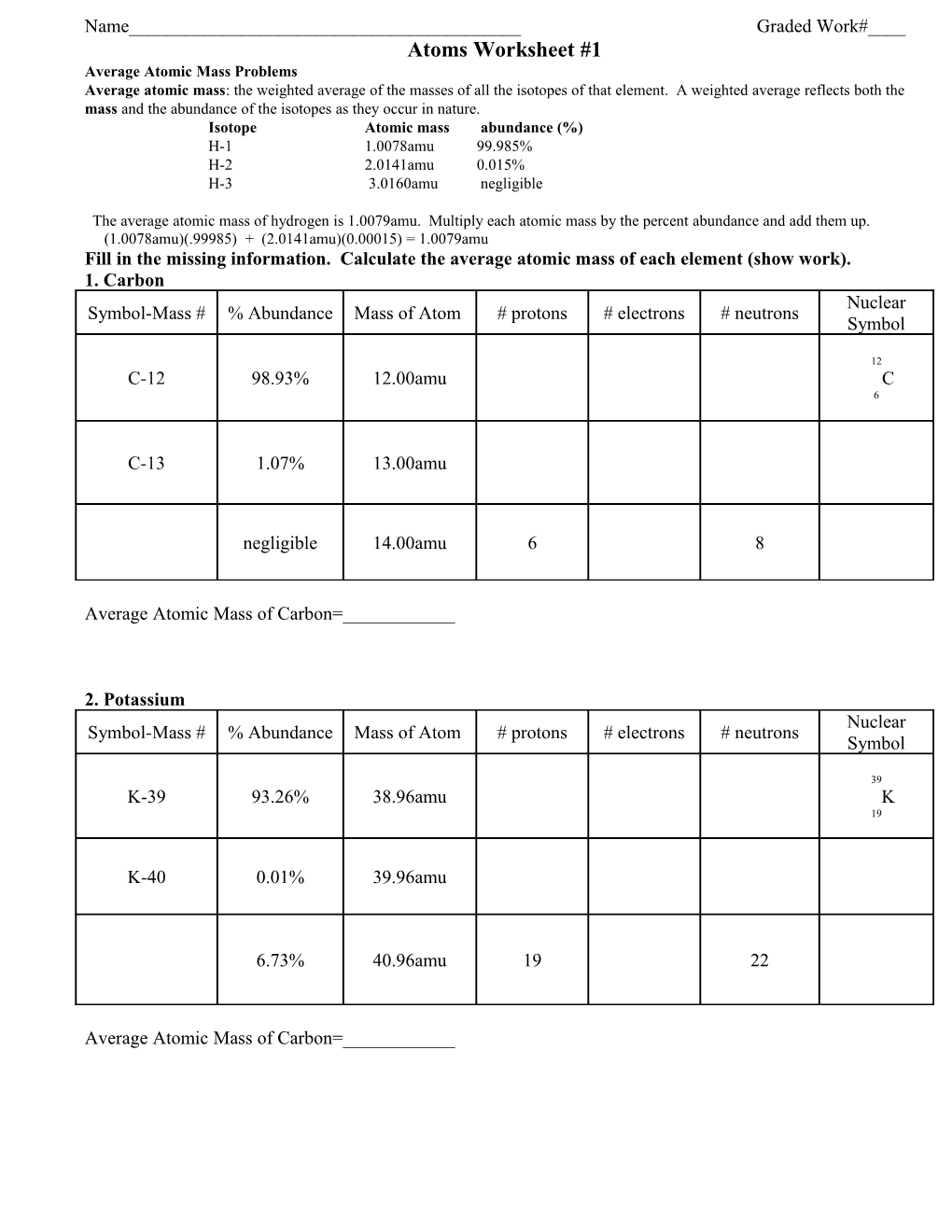
Name______Graded Work#____ Atoms Worksheet #1 Average Atomic Mass Problems Average atomic mass: the weighted average of the masses of all the isotopes of that element. A weighted average reflects both the mass and the abundance of the isotopes as they occur in nature. Isotope Atomic mass abundance (%) H-1 1.0078amu 99.985% H-2 2.0141amu 0.015% H-3 3.0160amu negligible
The average atomic mass of hydrogen is 1.0079amu. Multiply each atomic mass by the percent abundance and add them up. (1.0078amu)(.99985) + (2.0141amu)(0.00015) = 1.0079amu Fill in the missing information. Calculate the average atomic mass of each element (show work). 1. Carbon Nuclear Symbol-Mass # % Abundance Mass of Atom # protons # electrons # neutrons Symbol
12 C-12 98.93% 12.00amu C 6
C-13 1.07% 13.00amu
negligible 14.00amu 6 8
Average Atomic Mass of Carbon=______
2. Potassium Nuclear Symbol-Mass # % Abundance Mass of Atom # protons # electrons # neutrons Symbol
39 K-39 93.26% 38.96amu K 19
K-40 0.01% 39.96amu
6.73% 40.96amu 19 22
Average Atomic Mass of Carbon=______3. Nitrogen Nuclear Symbol-Mass # % Abundance Mass of Atom # protons # electrons # neutrons Symbol
N-14 99.632% 14.00amu
0.0368% 15.00amu 7 8
Average Atomic Mass of Carbon=______
4. Chlorine Nuclear Symbol-Mass # % Abundance Mass of Atom # protons # electrons # neutrons Symbol
Cl-35 75.78% 34.97amu
24.22% 36.97amu 17 20
Average Atomic Mass of Carbon=______
5. Oxygen Nuclear Symbol-Mass # % Abundance Mass of Atom # protons # electrons # neutrons Symbol
O-16 99.757% 15.99amu
O-17 0.038% 16.99amu
O-18 0.205% 18.00amu
Average Atomic Mass of Carbon=______
6. Silver Nuclear Symbol-Mass # % Abundance Mass of Atom # protons # electrons # neutrons Symbol
Ag-107 51.839% 106.91amu
48.161% 108.90amu 47 62
Average Atomic Mass of Carbon=______
7. Copper Nuclear Symbol-Mass # % Abundance Mass of Atom # protons # electrons # neutrons Symbol
Cu-63 69.17% 62.93amu
Cu-65 30.83% 64.93amu
Average Atomic Mass of Carbon=______
8. Counting Atoms – How many atoms are in the following compounds? a) CaCl2______b) NH4OH______c) NaCl______d) N2O7______
e) P2O5______f) Zn(NO3)2______g) Al2(CO3)3______h) 4 Na3PO4______
i) 3 Mg(NO3)2______j) 6 C6H12O6______k) 8 (NH4)2Cr2O7______
9. Element Z has 2 natural isotopes. One isotope has a of 15.0amu and has a relative abundance of 30%. The other isotope has a mass of 16.0amu and has a relative abundance of 70%. Estimate the average atomic mass for this element to one decimal place.
10. Three isotopes of argon occur in nature – Ar-36, Ar-38, Ar-40. Calculate the average atomic mass of argon to two decimal places, given the following relative atomic masses and abundances of each of the isotopes: Ar- 36 (35.97amu; 0.337%), Ar-38 (37.96amu; 0.063%), and Ar-40 (39.96amu; 99.600%).
11. Naturally occurring boron is 80.20% boron-11 (atomic mass = 11.01amu) and 19.80% of some other isotopic form of boron. What must the atomic mass of this second isotope be in order to account for the 10.81amu average atomic mass of boron? (Write the answer to two decimal places.)