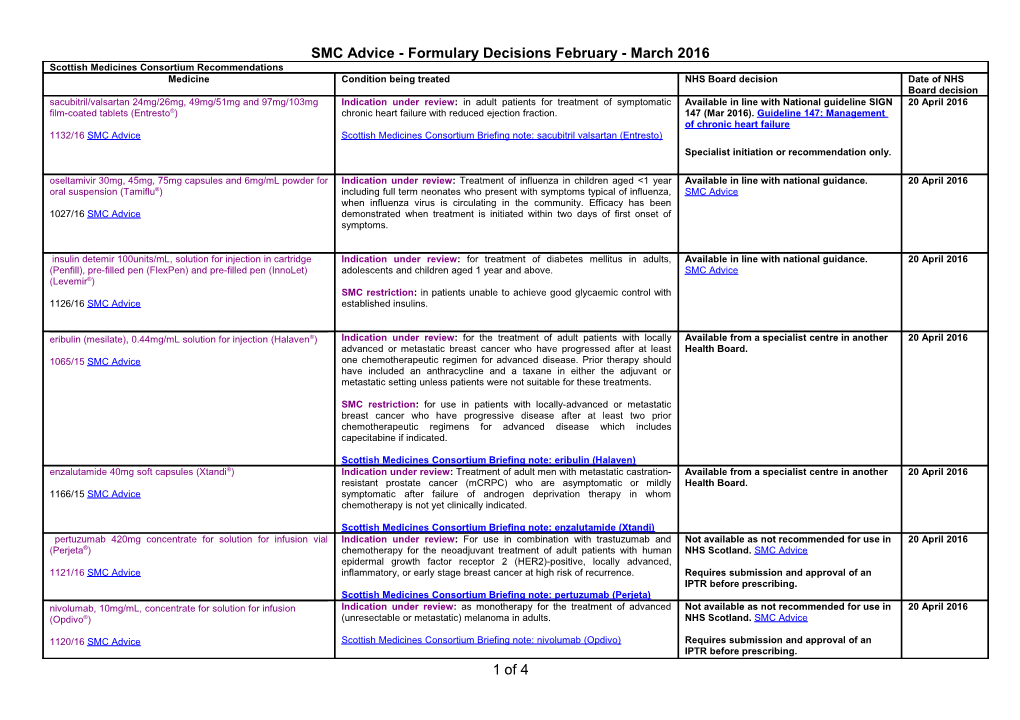
SMC Advice - Formulary Decisions February - March 2016 Scottish Medicines Consortium Recommendations Medicine Condition being treated NHS Board decision Date of NHS Board decision sacubitril/valsartan 24mg/26mg, 49mg/51mg and 97mg/103mg Indication under review: in adult patients for treatment of symptomatic Available in line with National guideline SIGN 20 April 2016 film-coated tablets (Entresto®) chronic heart failure with reduced ejection fraction. 147 (Mar 2016). Guideline 147: Management of chronic heart failure 1132/16 SMC Advice Scottish Medicines Consortium Briefing note: sacubitril valsartan (Entresto) Specialist initiation or recommendation only. oseltamivir 30mg, 45mg, 75mg capsules and 6mg/mL powder for Indication under review: Treatment of influenza in children aged <1 year Available in line with national guidance. 20 April 2016 oral suspension (Tamiflu®) including full term neonates who present with symptoms typical of influenza, SMC Advice when influenza virus is circulating in the community. Efficacy has been 1027/16 SMC Advice demonstrated when treatment is initiated within two days of first onset of symptoms.
insulin detemir 100units/mL, solution for injection in cartridge Indication under review: for treatment of diabetes mellitus in adults, Available in line with national guidance. 20 April 2016 (Penfill), pre-filled pen (FlexPen) and pre-filled pen (InnoLet) adolescents and children aged 1 year and above. SMC Advice (Levemir®) SMC restriction: in patients unable to achieve good glycaemic control with 1126/16 SMC Advice established insulins.
eribulin (mesilate), 0.44mg/mL solution for injection (Halaven®) Indication under review: for the treatment of adult patients with locally Available from a specialist centre in another 20 April 2016 advanced or metastatic breast cancer who have progressed after at least Health Board. 1065/15 SMC Advice one chemotherapeutic regimen for advanced disease. Prior therapy should have included an anthracycline and a taxane in either the adjuvant or metastatic setting unless patients were not suitable for these treatments.
SMC restriction: for use in patients with locally-advanced or metastatic breast cancer who have progressive disease after at least two prior chemotherapeutic regimens for advanced disease which includes capecitabine if indicated.
Scottish Medicines Consortium Briefing note: eribulin (Halaven) enzalutamide 40mg soft capsules (Xtandi®) Indication under review: Treatment of adult men with metastatic castration- Available from a specialist centre in another 20 April 2016 resistant prostate cancer (mCRPC) who are asymptomatic or mildly Health Board. 1166/15 SMC Advice symptomatic after failure of androgen deprivation therapy in whom chemotherapy is not yet clinically indicated.
Scottish Medicines Consortium Briefing note: enzalutamide (Xtandi) pertuzumab 420mg concentrate for solution for infusion vial Indication under review: For use in combination with trastuzumab and Not available as not recommended for use in 20 April 2016 (Perjeta®) chemotherapy for the neoadjuvant treatment of adult patients with human NHS Scotland. SMC Advice epidermal growth factor receptor 2 (HER2)-positive, locally advanced, 1121/16 SMC Advice inflammatory, or early stage breast cancer at high risk of recurrence. Requires submission and approval of an IPTR before prescribing. Scottish Medicines Consortium Briefing note: pertuzumab (Perjeta) nivolumab, 10mg/mL, concentrate for solution for infusion Indication under review: as monotherapy for the treatment of advanced Not available as not recommended for use in 20 April 2016 (Opdivo®) (unresectable or metastatic) melanoma in adults. NHS Scotland. SMC Advice
1120/16 SMC Advice Scottish Medicines Consortium Briefing note: nivolumab (Opdivo) Requires submission and approval of an IPTR before prescribing. 1 of 4 SMC Advice - Formulary Decisions February - March 2016 capsaicin (Qutenza®) 179mg cutaneous patch Indication under review: Treatment of peripheral neuropathic pain in Not available as not recommended for use in 20 April 2016 diabetic adults either alone or in combination with other medicinal products NHS Scotland. SMC Advice 1140/16 SMC Advice for pain. Requires submission and approval of an IPTR before prescribing for this indication. daptomycin (Cubicin®) powder for concentrate for solution for Indication under review: Treatment of paediatric (1 to 17 years of age) Not available as not recommended for use in 20 April 2016 injection or infusion patients with complicated skin and soft-tissue infections. NHS Scotland. SMC Advice
1141/16 SMC Advice Requires submission and approval of an IPTR before prescribing. camellia sinensis (green tea) leaf extract 10% ointment Indication under review: Cutaneous treatment of external genital and Available in line with local guidance. 20 April 2016 (Catephen®) perianal warts (condylomata acuminata) in immunocompetent patients from the age of 18 years. Restricted to 3rd line use after failure of 1133/16 SMC Advice podophyllotoxin and imiquimod or where SMC restriction: for use in patients not suitable for podophyllotoxin or who alternative agents are unsuitable. have not responded to treatment with podophyllotoxin.
Restricted to use by GUM clinic.
isavuconazole, 200mg powder for concentrate for solution for Indication under review: in adults for the treatment of: Not routinely available as local clinical 20 April 2016 infusion and 100mg hard capsules (Cresemba®) invasive aspergillosis experts do not wish to add the medicine to mucormycosis in patients for whom amphotericin B is inappropriate the formulary at this time. 1129/16 SMC Advice Fife Formulary Chapter 5 Infections alendronic acid 70mg effervescent tablet (Binosto®) Indication under review: Treatment of postmenopausal osteoporosis. Not routinely available as NHS Fife Board 20 April 2016 does not support Fife Formulary inclusion. 1137/16 SMC Advice SMC restriction: for use in patients who are unable to swallow tablets where alendronic acid is the appropriate treatment choice. Fife Formulary Chapter 6 Endocrine everolimus 2.5mg, 5mg and 10mg tablets (Afinitor®) Indication under review: For the treatment of hormone receptor-positive, Available from a specialist centre in another 20 April 2016 HER2/neu negative advanced breast cancer, in combination with Health Board. 872/13 SMC Advice exemestane, in postmenopausal women without symptomatic visceral disease after recurrence or progression following a non-steroidal aromatase inhibitor. ataluren 125mg, 250mg, 1,000mg granules for oral suspension Indication under review: Treatment of Duchenne muscular dystrophy Not available as not recommended for use in 20 April 2016 (Translarna®) resulting from a nonsense mutation in the dystrophin gene, in ambulatory NHS Scotland. SMC Advice patients aged 5 years and older. 1131/16 SMC Advice Requires submission and approval of an IPTR before prescribing. eculizumab 300mg/30mL vial concentrate for solution for Indication under review: In adults and children, for the treatment of patients Not available as not recommended for use in 20 April 2016 infusion (Soliris®) with paroxysmal nocturnal haemoglobinuria (PNH). Evidence of clinical NHS Scotland. SMC Advice benefit is demonstrated in patients with haemolysis with clinical symptom(s) 1130/16 SMC Advice indicative of high disease activity, regardless of transfusion history. Requires submission and approval of an IPTR before prescribing. 2 of 4 SMC Advice - Formulary Decisions February - March 2016
3 of 4 SMC Advice - Formulary Decisions February - March 2016
Summary of Approved Lothian Formulary Committee Decisions for SCAN Medicines Product Name SMC Advice Place in therapy Lothian formulary Committee Decision Add to Fife Formulary Yes / No Rationale lipegfilgrastim lipegfilgrastim (Lonquex®) is accepted for restricted use In adult patients suitable for primary or Included on the additional list. Yes (Lonquex®) within NHS Scotland. secondary prophylaxis against neutropenic 908/13 sepsis with GCSF. Specialist hospital use only. Indication under review: Reduction in the duration of neutropenia and the incidence of febrile neutropenia in Will replace pegfilgrastim as the long acting adult patients treated with cytotoxic chemotherapy for GCSF product of choice. malignancy (with the exception of chronic myeloid leukaemia and myelodysplastic syndromes).
SMC restriction: where a long-acting granulocyte-colony- stimulating factor is appropriate.
In a randomised, double-blind study, in adults with breast cancer given myelosuppressive chemotherapy associated with a high risk of febrile neutropenia, lipegfilgrastim was compared with another long-acting granulocyte colony- stimulating factor when used as primary prophylaxis against febrile neutropenia. The study found lipegfilgrastim was non-inferior to the comparator preparation in terms of the mean duration of severe neutropenia in the first chemotherapy cycle.
4 of 4