CHM 1025C & CHM 1032C Labs Spectroscopy: Electron Energy Levels-----The Bohr Atom
OBJECTIVES To distinguish between a continuous spectrum and a line spectrum. To understand the relationship between spectral lines and electron energy levels. To identify the unknown element in a fluorescent light by its “atomic fingerprint.” To compare the observed wavelengths of the lines in the hydrogen spectrum to the calculated wavelengths. To become proficient in using a hand spectroscope.
DISCUSSION
On December 14, 1900, a revolutionary concept was born. The concept came from the mind of Max Planck, a German professor of physics. The concept was quite simple: since matter comes in lumps, perhaps energy also comes in lumps. We call a lump of matter an atom; Planck called a lump of radiant energy a quantum. The concept that light energy comes in discrete packets was revolutionary because previously light had been viewed as a continuous wave of energy.
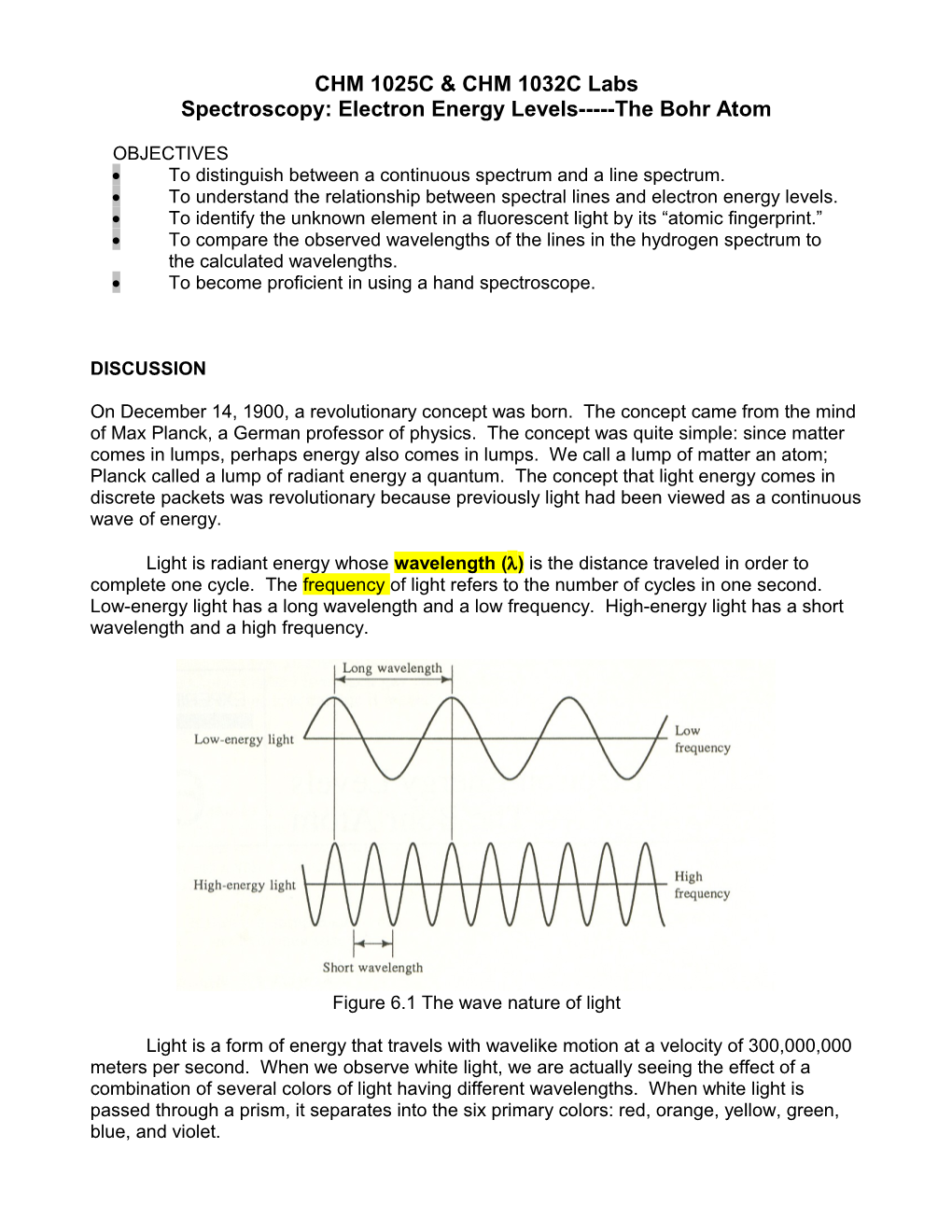
Light is radiant energy whose wavelength () is the distance traveled in order to complete one cycle. The frequency of light refers to the number of cycles in one second. Low-energy light has a long wavelength and a low frequency. High-energy light has a short wavelength and a high frequency.
Figure 6.1 The wave nature of light
Light is a form of energy that travels with wavelike motion at a velocity of 300,000,000 meters per second. When we observe white light, we are actually seeing the effect of a combination of several colors of light having different wavelengths. When white light is passed through a prism, it separates into the six primary colors: red, orange, yellow, green, blue, and violet. Figure 6.2 The six component colors of white light
A rainbow is a natural phenomenon created when sunlight passes through raindrops. Individual drops of rain act as miniature prisms to separate sunlight into various bands of color. The wavelength of light can be expressed in nanometers, where a nanometer (nm) is one-billionth of a meter. The range of visible light, violet to red, is usually considered 400-700 nm. However, in this experiment, you will probably observe visible colors from about 450-650 nm.
Visible light is only a small portion of the entire spectrum of radiant energy. Notice in Figure 6.3 that visible light is a small window in a vast spectrum of radiant energy. This spectrum includes X ray, ultraviolet, visible, infrared, and microwave radiation. Our eyes can only detect light in the visible spectrum and not in other regions. That is, the wavelength of ultraviolet light is too short to be visible and wavelength of infrared light is too long to be seen by the human eye.
Figure 6.3 The continuous range of light energy from gamma rays to radio waves is called the radiant energy spectrum, or sometimes the electromagnetic spectrum.
The Bohr Model of the Atom
In 1911 Ernest Rutherford and his fellow researchers were investigating the scattering of high-energy alpha particles by a thin gold foil. Much to their amazement a few particles were deflected at severe angles and a very few actually bounced off the gold foil. This startling result revealed that the atom contains a dense positively charged nucleus surrounded by electrons. Rutherford suggested that the electrons revolve about the nucleus as the planets revolve around the sun. This became known as the planetary model of the atom. A few months later the Danish physicist Niels Bohr joined Rutherford in England. Bohr adopted the planetary model of the atom but went further and speculated that electrons travel in circular orbits at a fixed distance from the nucleus (figure 6.4). Since the orbit distance is fixed, the electron possesses a definite energy. Moreover, Bohr proposed that the electron could change energy by jumping from level to level.
Figure 6.4 In the Bohr model of the atom, the electron must occupy a discrete orbit of fixed energy levels. Electron Energy Levels The Bohr model was a beautiful mental picture of electrons in atoms. It was consistent with Planck’s quantum principle, but nobody knew whether the model was right or wrong. There simply was no experimental evidence to support the theory. In 1913 Bohr received a research paper on the emission of light from electrically excited hydrogen gas. Bohr noticed that hydrogen emitted only discrete wavelengths of light and not a continuous spectrum. The three most prominent emission lines were violet, blue-green and red. We can describe the experiment as follows. First, hydrogen gas is sealed in a glass tube. Then, an electrical voltage is applied to energize the hydrogen gas. An instant later the excited hydrogen atoms release energy in the form of light. When this light passes through a prism, it separates into narrow bands of light having a specific wavelength. Figure 6.5 illustrates the emission spectrum of hydrogen.
Figure 6.5 In the emission spectrum of hydrogen the light from the gas discharge tube appears reddish-purple. After the light passes through a prism the reddish- purple splits into three lines which are violet, blue-green, and red.
As Bohr reflected on the emission spectrum of hydrogen, he realized that he had powerful experimental for his model of the atom. His concept of energy levels was supported by the line spectrum of hydrogen. He reasoned as follows: When a voltage is applied to a hydrogen gas discharge tube, electrons jump to a higher energy level. For example, the electron may jump from the first level to the second, third, fourth, or fifth level. The electron is excited in a higher level and can lose energy by dropping to any one of the lower levels closer to the nucleus. When the electron drops to a lower energy level, it loses a specific amount of energy that corresponds to one quantum of light. That is, the quantum of light possesses the same amount of energy as that lost by the electron as it drops from a higher to lower energy level. This experimental evidence supported Bohr’s model of the atom perfectly. However, it was Planck’s idea that light comes in lumps that allowed for Bohr’s penetrating insight. Figure 6.6 shows the correlation between Bohr’s energy levels and observed lines in the hydrogen spectrum.
Figure 6.6 The relationship between quantum levels and hydrogen spectral lines is shown. The violet is emitted when electrons drop from the 5th to the 2nd orbit. The blue- green line corresponds to the transition from the 4th to 2nd; the red line when electrons drop from the 3rd to 2nd quantum level.
Further study of emission spectra revealed that each element produces a different set of spectral lines. Thus, the energy difference between energy levels is unique for atoms of each element. For this reason, the line spectrum of a given element is unique and is sometimes referred to as an “atomic fingerprint.” The Balmer Formula
Jakob Balmer, a Swiss mathematician and physicist, did not publish his first paper until he was sixty years old. In 1885, Balmer published a mathematical formula that accounted for the visible lines emitted from excited hydrogen gas. The Balmer formula shows that the wavelength () of light for each hydrogen spectral line is related to a small whole number (n) in the following way:
When Balmer set n = 3, the calculated wavelength was equal to the wavelength of the red line in the hydrogen spectrum. Substituting n = 4 and n = 5 gave values for the blue-green and violet lines observed in the spectrum of hydrogen. When Niels Bohr read Balmer’s paper, he realized that the n value could represent an energy level and that the 2 in the formula corresponded to the second energy level.
The following sample calculation uses the Balmer formula to find the wavelength of a spectral line in the Hydrogen spectrum.
Example Exercise 6.1 Calculate the wavelength of visible light corresponding to the energy released when the electron drops from n = 6 to n = 2 in a hydrogen atom.
Solution: We can use the Balmer formula to calculate the wavelength of this visible spectral line as follows: A spectral line of this wavelength is in the violet portion of the spectrum. Although this line is not intense, if you look very carefully at the emission spectrum of hydrogen, you may see a faint violet line at 410 nm.
The Rydberg Equation
Several years after Balmer had introduced his formula, the Swedish physicist Johannes Rydberg derived a more general formula for calculating the wavelengths of spectral lines. The Rydberg equation accounts for an electron dropping from any higher energy level (nH) to any lower level (nL). The general form of the equation is:
The Balmer formula is adequate to explain the visible lines in the spectrum of hydrogen because they correspond to excited electrons dropping to n = 2. However, the Rydberg equation also accounts for spectral lines in the ultraviolet and infrared portions of the spectrum. When the electron drops to n = 1, the spectral lines are in the ultraviolet region of the spectrum and therefore not visible to the eye. Similarly, when the electron falls to n = 3, the spectral lines are in the infrared region of the spectrum and not visible.
The Quantum Mechanical Atom
In the mid-1920s an entirely new model of the atom began to emerge. The behavior of electrons in atoms could no longer be adequately explained using the Bohr model of the atom. The German physicist Werner Heisenberg proposed that it was impossible to determine accurately both the position and energy of an electron. Heisenberg stated that it is impossible to precisely measure both the location and momentum of a small particle simultaneously. In fact, the more accurately the position of an electron in an atom is known, the less precisely its energy can be calculated. Heisenberg’s statement came to be known as the uncertainty principle.
Gradually, the deeper nature of the atom came into focus. The new model retained the idea of energy levels but incorporated the concept of uncertainty. The new model that emerged became known as the quantum mechanical atom. In the Bohr model, the energy of an electron is defined in terms of a stationary orbit about the nucleus. In the quantum mechanical model, the energy of an electron is described in terms of its probability of being found within a prescribed volume of space surrounding the nucleus. This region of high probability for finding an electron of given energy is called an orbital. In the quantum mechanical model, an orbital describes the energy state of the electron.
EQUIPMENT AND CHEMICALS Science Kit Hand Spectroscope
[The SK spectroscope (WW16525M00, ~$9.00) is available from Science Kit, Inc., 777 East Park Drive, Tonawanda, NY 14150 (800) 828-7777.] spectrum tube power supply gas discharge tubes: helium, neon, argon, krypton, xenon, mercury, hydrogen colored pencils: violet, blue, green, yellow, orange, red (optional)
Figure: The Hand Spectroscope. Light enters the spectroscope through the slit and strikes the diffraction grating. The grating is a thin piece of plastic etched with hundreds of parallel grooves. The diffraction grating separates light into different wavelengths. The numbers on the scale represent wavelengths from 400 nm to 700 nm. The digit 4 is read as 400nm, 5 as 500 nm and 6 as 600 nm. There are ten divisions between each number on the scale; therefore each division is 10 nm. For example, the scale divisions between 4 and 5 are read as: 410, 420, 430, 440, 450, 460, 470, 480, and 490 nm.
PROCEDURE
A. Continuous Spectrum vs. Line Spectrum
1. With the hand spectroscope observe the emission spectrum from one or more of the following: the sun, an ordinary light bulb, the light reflected off an overhead projector screen. Color the observed spectrum onto the chart in the Data Table.
2. Insert a helium gas discharge tube into a power supply and observe and record the color of light produced. Carefully examine the emitted light from the helium using the spectroscope. Mark the wavelength of the six most intense lines in the Data Table. Note: It is necessary to have room lights on in order read the scale divisions in the spectroscope.
B. Identifying an Element by its “Atomic Fingerprint”
1. Place a neon gas discharge tube into the power supply and describe and record the observed color. Examine the emitted light through the spectroscope and record in the Data the color all intense lines (V, B, G, Y, O, R). Replace the neon tube with each of the following gas discharge tubes: argon, krypton, xenon, and mercury. Record the observed color as well as the color of the intense lines in each spectrum. Note: The spectral lines are best viewed in the dark. Room lights can be turned off since scale readings are not necessary. It may be convenient to turn on an overhead projector light long enough to record the data.
2. Observe the line spectrum from a fluorescent light using the spectroscope. Disregard the continuous background spectrum and draw the three most intense lines in the Data Table. Compare the line spectrum from the fluorescent light to the lines from the Ne, Ar, Kr, Xe, and Hg gas discharge tubes. Identify the unknown element in the fluorescent light from its “atomic fingerprint.”
C. Hydrogen Emission Spectrum
1. Closely observe the emission spectrum of hydrogen with a hand spectroscope. Record the wavelengths of the three most intense lines in the Data Table. (Room light is once again necessary to read the scale.)
2. Using the Balmer formula, calculate the wavelength () of light produced when an electron drops from the 3rd to 2nd energy level. (Round the answer to two significant digits; for example, 433.3 nm rounds to 430 nm.)
3. Repeat the wavelength calculation for the spectral lines produced when the electron drops from the 4th to 2nd energy level; and from the 5th to 2nd energy level.
4. Record the observed and calculated wavelength values in the Data Table. Calculate the error by finding the difference between the observed and calculated wavelengths. Spectroscopy: PRE-LABORATORY ASSIGNMENT
1. In you own words, define the following terms: “Atomic fingerprint”______Balmer formula______continuous spectrum______frequency______light______line spectrum______nanometer (nm)______quantum______visible spectrum______wavelength______
2. Name the six primary colors in the visible spectrum in order of: (a) decreasing wavelength______(b) decreasing frequency______(c) decreasing energy______
3. What wavelengths of light are indicated by the following scale readings observed through a hand spectroscope?
4. What are the colors of the three most intense lines in the emission spectrum of hydrogen? 5. A hydrogen gas discharge tube emits a faint violet line when electrons drop from the 7th to the 2nd energy level. Use the Balmer formula to calculate the wavelength for this spectral line.
6. How many quanta of light are emitted when 1 electron drops from the 7th to 2nd energy level? When 10 electrons drop from the 5th to 2nd energy level?
7. What safety precautions must be observed in this experiment? Spectroscopy: NAME ______
DATE ______SECTION ______
DATA TABLE Spectroscope # ______
A. Continuous Spectrum vs. Line Spectrum 1. Continuous Emission Spectrum - White Light
2. Line emission spectrum - Helium
B. Identifying an Element by its “Atomic Fingerprint”
1. Line Spectra from Gas Discharge Tubes (a) Neon Observed Color: ______Spectral Lines: V B G Y O R
(b) Argon Observed Color: ______Spectral Lines: V B G Y O R
(c) Krypton Observed Color: ______Spectral Lines: V B G Y O R
(d) Xenon Observed Color: ______Spectral Lines: V B G Y O R
(e) Mercury Observed Color: ______Spectral Lines: V B G Y O R
2. Line Spectrum from a Fluorescent Light
The unknown element in the fluorescent light is ______
C. Hydrogen Emission Spectrum 1. Observed Wavelengths of Spectral Lines
2. Calculated Wavelengths of Spectral Lines
(a) red line (electrons drop from 3rd level to 2nd level)
(b) blue-green line (electrons drop from 4th level to 2nd level)
(c) violet line (electrons drop from 5th level to 2nd level) Spectral Line Observed Calculated Difference in Wavelength Wavelength Wavelengths red line ______nm ______nm ______nm blue-green line ______nm ______nm ______nm violet line ______nm ______nm ______nm