1 Electronic Supplementary Material 2 3Simultaneous determination of cadmium(II), lead(II) and copper(II) by using a 4screen-printed electrode modified with mercury nano-droplets
5 Wei Song · Lei Zhang · Lei Shi · Da-wei Li · Yang Li · Yi-tao Long 6
7Fabrication of SPE
8Silver ink acting as conductive medium was printed as the first layer and cured at 100 9°C for 20 min. The circular working electrode (planar area: 3.1 mm2) was fabricated 10from a carbon ink followed by heat treating at 100 °C for 20 min. The reference 11electrode was printed with 40 % silver chloride in silver paste, and the auxiliary 12electrode was printed with carbon ink. The insulating ink was used finally to provide 13an insulation layer defining the working electrode surface area. The SPE was 14pretreated in a 0.1 M acetic acid buffer (pH 5.0) containing 0.1 M KCl by applying an 15anodic potential of 1.7 V for 3 min before use [1].
16Behavior of mercury adsorption
17
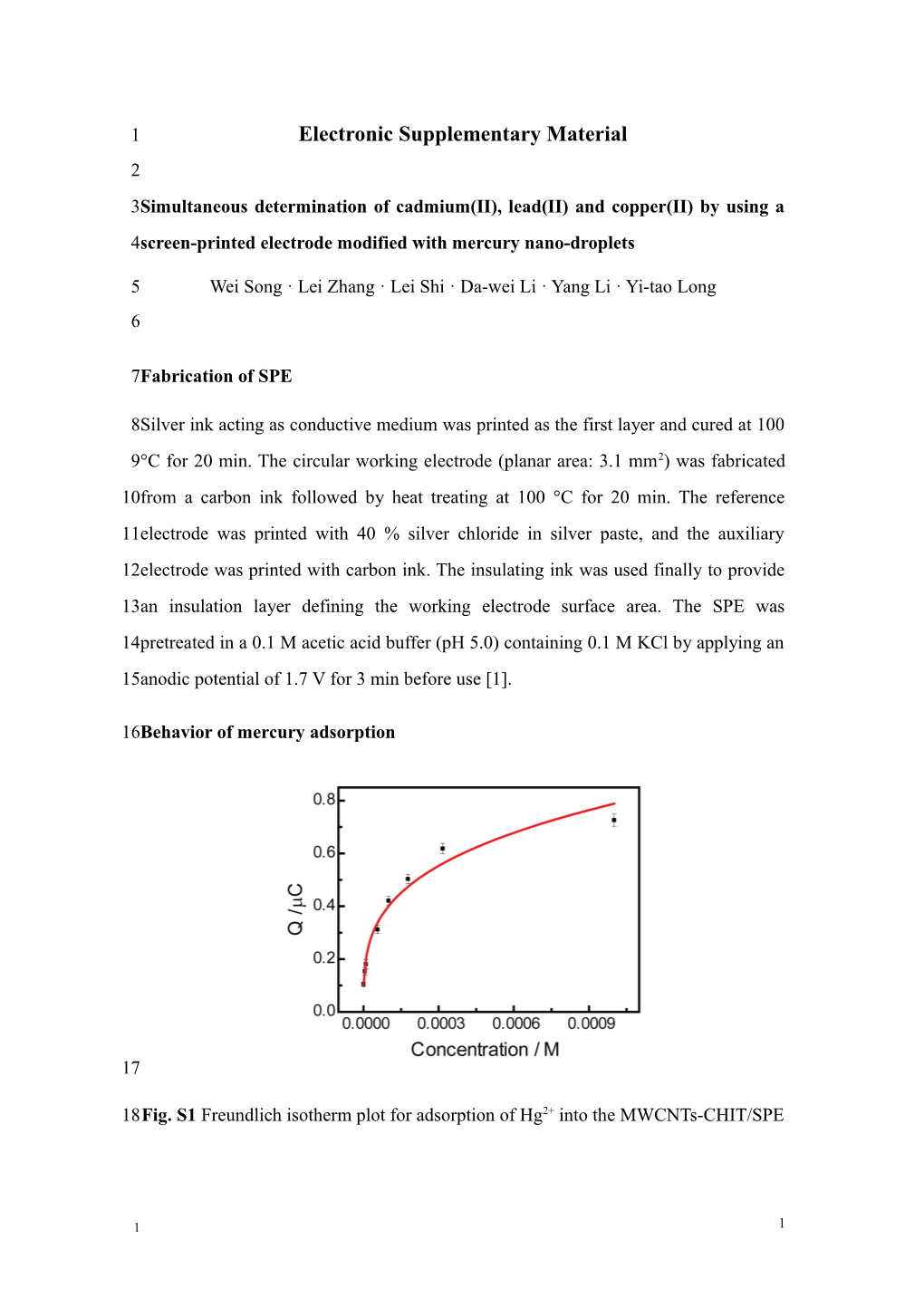
18Fig. S1 Freundlich isotherm plot for adsorption of Hg2+ into the MWCNTs-CHIT/SPE
1 1 1Equation 1 is the well-known Freundlich isotherm, which describes heterogeneous 2systems. It is an empirical equation and can be written as follows: 3 Q=KC1/n (1) 4where K is the Freundlich constant, which is indicative of the extent of adsorption, C 5is the concentration of Hg2+ and 1/n is the heterogeneity factor, an indicator of 6adsorption effectiveness [2].
7Effect of deposition potential and time
8
9 Fig. S2 Effect of deposition potential (a) and time (b) on peak current
1 2 1 2Voltammetric behavior of these three metal ions on mercury modified MWCNT- 3CHIT/SPE was evaluated in terms of their influence of stripping parameters, such as 4the effect of deposition potential and deposition time. Effect of deposition potential 5was investigated in the range of −0.90 to −1.30 V. 6 Fig. S2a shows that the sensitivity got improved when the more negative potential 7was applied. Because excess negative potential may cause much fouling and the 8evolution of hydrogen gas at the working electrode, −1.20 V was chosen. The effect of 9the deposition time on the stripping peak current was examined in the range of 1030−600 s (Fig. S2b). Taking into account of the sensitivity and the time consumption, 11we chose 300 s as the accumulation time in our experiments. 12 13
14References
151. Wang J, Pedrero M, Sakslund H, Hammerich O, Pingarron J (1996) 16 Electrochemical activation of screen-printed carbon strips, Analyst 21: 345 172. Cheng R, Ou SJ, Xiang B, Li YJ, Liao QQ (2009) Equilibrium and Molecular 18 Mechanism of Anionic Dyes Adsorption onto Copper(II) Complex of 19 Dithiocarbamate-Modified Starch, Langmuir 26: 752
1 3