Part 1: Introduction to organic Chemistry
Many carbon compounds are found in living organisms which is why their study got the name organic chemistry. Today, organic chemistry includes all carbon compounds whatever their origin, except CO, CO2 and the carbonates, which traditionally are included in inorganic chemistry.
Why carbon?
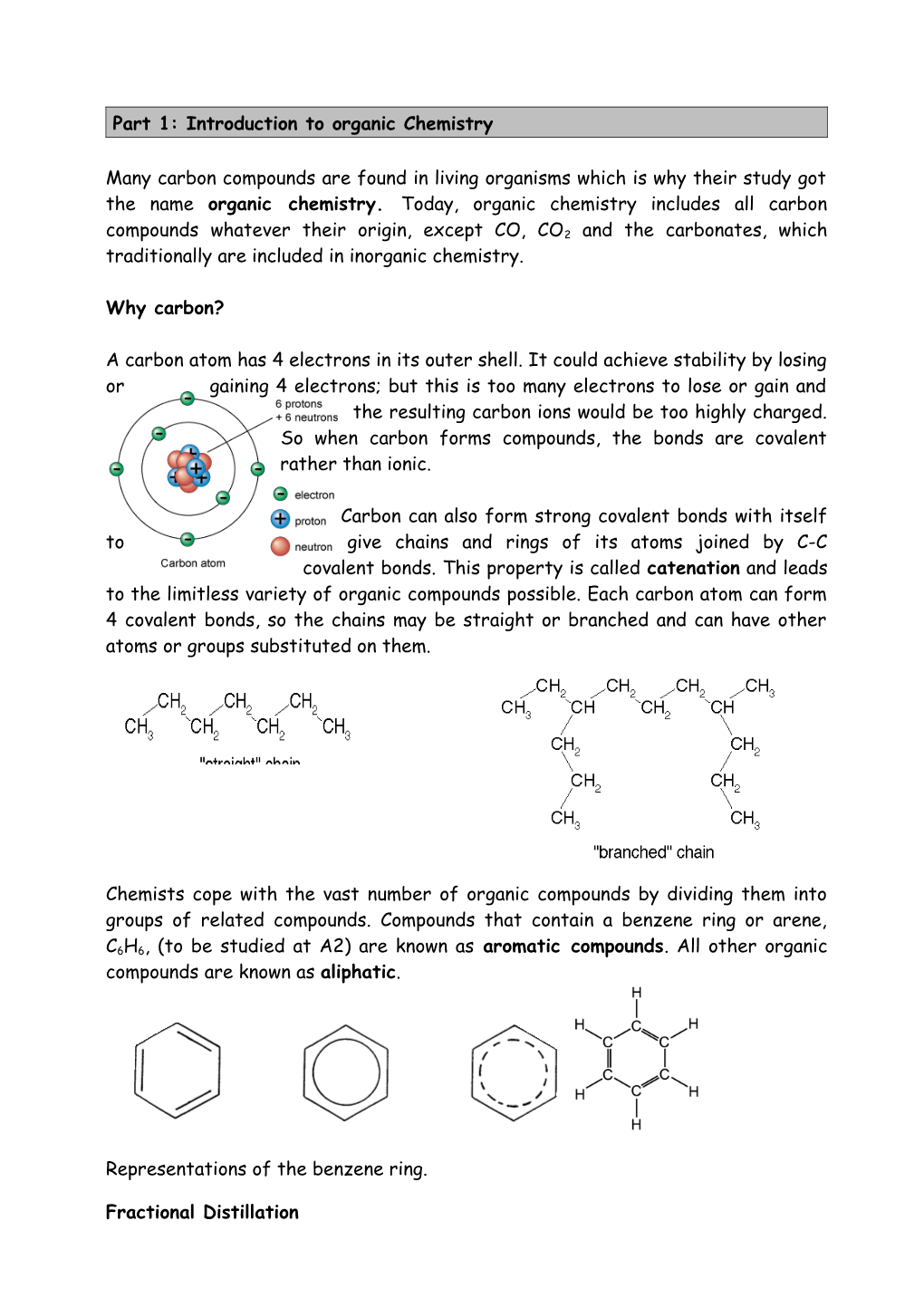
A carbon atom has 4 electrons in its outer shell. It could achieve stability by losing or gaining 4 electrons; but this is too many electrons to lose or gain and the resulting carbon ions would be too highly charged. So when carbon forms compounds, the bonds are covalent rather than ionic.
Carbon can also form strong covalent bonds with itself to give chains and rings of its atoms joined by C-C covalent bonds. This property is called catenation and leads to the limitless variety of organic compounds possible. Each carbon atom can form 4 covalent bonds, so the chains may be straight or branched and can have other atoms or groups substituted on them.
Chemists cope with the vast number of organic compounds by dividing them into groups of related compounds. Compounds that contain a benzene ring or arene,
C6H6, (to be studied at A2) are known as aromatic compounds. All other organic compounds are known as aliphatic.
Representations of the benzene ring.
Fractional Distillation Crude oil, also called petroleum, is a complex mixture of hydrocarbons (compounds composed only of carbon and hydrogen atoms), which exist as a liquid in the earth's crust. Crude oil has many compositions; some is black, thick and tar like, while other crude oils are lighter in colour and thinner. The carbon and hydrogen atoms in crude oil are thought to have originated from the remains of microscopic marine organisms that were deposited at the bottom of seas and oceans and were transformed at moderately high temperature and high pressure into crude oil and natural gas.
In its raw form Crude Oil is not very useful. It must first be transported to a refinery where it is processed. The fundamental process is fractional distillation.
Fractional distillation is the process of partially separating the many compounds present in crude petroleum. The principle used is that the longer the carbon chain, the higher the temperature at which the compounds will boil. (N.B. branched chains have lower boiling points than straight chain molecules.) The crude petroleum is heated and changed into a gas. The gases are passed through a distillation column which becomes cooler as the height increases. When a compound in the gaseous state cools below its boiling point, it condenses into a liquid. The liquid fractions may be drawn off the distilling column at various heights.
(N.B. kerosene is often called paraffin.) Although all fractions of petroleum find uses, the greatest demand is for petrol (gasoline). One barrel of crude petroleum contains only 25-35% petrol. Transportation demands require that over 50% of the crude oil be converted into petrol. To meet this demand some petroleum fractions must be converted to petrol. This may be done by "cracking" - breaking down large molecules of heavy heating oil or "reforming" - changing molecular structures of low quality petrol molecules. We will look at “cracking” later.
Alkanes Many of the compounds in Crude Oil belong to a family or homologous series of compounds known as the alkanes. The alkanes are saturated molecules who share the same general formula: CnH2n+2.
Homologous series: a series or family of organic compounds with the same
functional group, whose members differ only in the addition of a CH2 group.
Functional group: the specific atom or group of atoms that confers a particular chemical property on a molecule, e.g. the –OH group in ethanol.
Saturated: the molecule contains the maximum amount of hydrogen atoms possible, with no double or triple bonds between atoms.
Task 2 The table below shows the formulae of some simple alkanes. Can you name them?
Name Formula Name Formula
CH4 C6H14
C2H6 C7H16
C3H8 C8H18
C4H10 C9H20
C5H12 C10H22 Isomerism All the alkanes with 4 or more carbon atoms in them show structural isomerism. This means that there are two or more different structural formulae that you can draw for each molecular formula.
For example, C4H10 could be either of these two different molecules:
These are called respectively butane and 2-methylpropane. Cycloalkanes Cycloalkanes again only contain carbon-hydrogen bonds and carbon-carbon single bonds, but this time the carbon atoms are joined up in a ring. The smallest cycloalkane is cyclopropane. If you count the carbons and hydrogens, you will see that they no longer fit the general formula CnH2n+2. By joining the carbon atoms in a ring, you have had to lose two hydrogen atoms.
The general formula for a cycloalkane is CnH2n. (This is the same as another homologous series known as the alkenes.) Don't imagine that these are all flat molecules. All the cycloalkanes from cyclopentane upwards exist as "puckered rings". Cyclohexane, for example, has a ring structure which looks like this:
This is known as the "chair" form of cyclohexane - from its shape which vaguely resembles a chair. Naming the alkanes A modern organic name is simply a code. Each part of the name gives you some useful information about the compound. For example, to understand the name 2-methylpropan-1-ol you need to take the name to pieces. The prop in the middle tells you how many carbon atoms there are in the longest chain (in this case, 3). The an which follows the "prop" tells you that there aren't any carbon-carbon double bonds. The other two parts of the name tell you about interesting things which are happening on the first and second carbon atom in the chain. Any name you are likely to come across can be broken up in this same way. Counting the carbon atoms You will need to remember the codes for the number of carbon atoms in a chain. There is no easy way around this - you have got to learn them. If you don't do this properly, you won't be able to name anything! Types of carbon-carbon bonds Whether or not the compound contains a carbon-carbon double bond is shown by the two letters immediately after the code for the chain length.
code means
an only carbon-carbon single bonds
en contains a carbon-carbon double bond
For example, butane means four carbons in a chain with no double bond. Propene means three carbons in a chain with a double bond between two of the carbons.
Alkyl groups
Compounds like methane, CH4, and ethane, CH3CH3, are members of a family of compounds called alkanes. If you remove a hydrogen atom from one of these you get an alkyl group. For example:
A methyl group is CH3.
An ethyl group is CH3CH2. These groups must, of course, always be attached to something else. Worked example: Write the structural formula for 2-methylpentane. Start decoding the name from the bit that counts the number of carbon atoms in the longest chain - pent counts 5 carbons. Are there any carbon-carbon double bonds? No - an tells you there aren't any. Now draw this carbon skeleton:
Put a methyl group on the number 2 carbon atom: Does it matter which end you start counting from? No - if you counted from the other end, you would draw the next structure. That's exactly the same as the first one, except that it has been flipped over.
Finally, all you have to do is to put in the correct number of hydrogen atoms on each carbon so that each carbon is forming four bonds.
If you had to name this yourself: Count the longest chain of carbons that you can find. Don't assume that you have necessarily drawn that chain horizontally. 5 carbons means pent. Are there any carbon-carbon double bonds? No - therefore pentane. There's a methyl group on the number 2 carbon - therefore 2- methylpentane. Why the number 2 as opposed to the number 4 carbon? In other words, why do we choose to number from this particular end? The convention is that you number from the end which produces the lowest numbers in the name - hence 2- rather than 4-. Task 1 1. Write the structural formula for 2,3-dimethylbutane.
2. Write the structural formula for 2,2-dimethylbutane.
3. Write the structural formula for 3-ethyl-2-methylhexane. If you had to name this yourself: How do you know what order to write the different alkyl groups at the beginning of the name? The convention is that you write them in alphabetical order - hence ethyl comes before methyl which in turn comes before propyl. The cycloalkanes In a cycloalkane the carbon atoms are joined up in a ring - hence cyclo. Worked example: Write the structural formula for cyclohexane. hexan shows 6 carbons with no carbon-carbon double bonds. cyclo shows that they are in a ring. Drawing the ring and putting in the correct number of hydrogens to satisfy the bonding requirements of the carbons gives:
Molecular formulae A molecular formula simply counts the numbers of each sort of atom present in the molecule, but tells you nothing about the way they are joined together.
For example, the molecular formula of butane is C4H10, and the molecular formula of ethanol is C2H6O. Molecular formulae are very rarely used in organic chemistry, because they don't give any useful information about the bonding in the molecule. About the only place where you might come across them is in equations for the combustion of simple hydrocarbons, for example:
In cases like this, the bonding in the organic molecule isn't important. Structural formulae A structural formula shows how the various atoms are bonded. There are various ways of drawing this and you will need to be familiar with all of them. Displayed formulae (or full structural formulae) This shows all the bonds in the molecule as individual lines. You need to remember that each line represents a pair of shared electrons. For example, this is a model of methane together with its displayed formula:
Notice that the way the methane is drawn bears no resemblance to the actual shape of the molecule. Methane isn't flat with 90° bond angles. The commonest way to draw structural formulae For anything other than the most simple molecules, drawing displayed formulae is a bit of a bother - especially all the carbon-hydrogen bonds. You can simplify the formula by writing, for example, CH3 or CH2 instead of showing all these bonds. So for example, ethanoic acid could be shown as:
You could even condense it further to CH3COOH, and would probably do this if you had to write a simple chemical equation involving ethanoic acid. You do, however, lose something by condensing the acid group in this way, because you can't immediately see how the bonding works. The syllabus states that: In candidates’ answers, an acceptable response to a request for a structural formula will be to give the minimal detail, using conventional groups, for an unambiguous structure, e.g. CH3CH2CH2OH for propan-1-ol, not C3H7OH. How to draw structural formulae in 3-dimensions There are occasions when it is important to be able to show the precise 3-D arrangement in parts of some molecules. To do this, the bonds are shown using conventional symbols: For example, you might want to show the 3-D arrangement of the groups around the carbon which has the -OH group in butan-2-ol. Butan-2-ol has the structural formula:
Using conventional bond notation, you could draw it as, for example:
Skeletal formulae In a skeletal formula, all the hydrogen atoms are removed from carbon chains, leaving just a carbon skeleton with functional groups attached to it. For example, we've just been talking about butan-2-ol. The normal structural formula and the skeletal formula look like this:
In a skeletal diagram of this sort there is a carbon atom at each junction between bonds in a chain and at the end of each bond (unless there is something else there already - like the -OH group in the example); there are enough hydrogen atoms attached to each carbon to make the total number of bonds on that carbon up to 4. Beware! Diagrams of this sort take practice to interpret correctly - and may well not be acceptable to your examiners (see below). There are, however, some very common cases where they are frequently used. These cases involve rings of carbon atoms which are surprisingly awkward to draw tidily in a normal structural formula.
Cyclohexane, C6H12, is a ring of carbon atoms each with two hydrogens attached. This is what it looks like in both a structural formula and a skeletal formula. And this is cyclohexene, which is similar but contains a double bond:
But the commonest of all is the benzene ring, C6H6, which has a special symbol of its own. Part 2: Structural Isomerism
What are isomers? Isomers are molecules that have the same molecular formula, but have a different arrangement of the atoms in space. That excludes any different arrangements which are simply due to the molecule rotating as a whole, or rotating about particular bonds. For example, both of the following are the same molecule. They are not isomers. Both are butane.
There are also endless other possible ways that this molecule could twist itself. There is completely free rotation around all the carbon-carbon single bonds. If you had a model of a molecule in front of you, you would have to take it to pieces and rebuild it if you wanted to make an isomer of that molecule. If you can make an apparently different molecule just by rotating single bonds, it's not different - it's still the same molecule. What are structural isomers? In structural isomerism, the atoms are arranged in a completely different order. This is easier to see with specific examples. What follows looks at some of the ways that structural isomers can arise. The names of the various forms of structural isomerism probably don't matter all that much, but you must be aware of the different possibilities when you come to draw isomers. Types of structural isomerism Chain isomerism These isomers arise because of the possibility of branching in carbon chains. For example, there are two isomers of butane, C4H10. In one of them, the carbon atoms lie in a "straight chain" whereas in the other the chain is branched. Be careful not to draw "false" isomers which are just twisted versions of the original molecule. For example, this structure is just the straight chain version of butane rotated about the central carbon-carbon bond. You could easily see this with a model. This is the example we've already used at the top of this page.
Pentane, C5H12, has three chain isomers. If you think you can find any others, they are simply twisted versions of the ones below.
Task 1: Can you name and draw the isomers of pentane, C5H12 below?
Task 2: How many isomers of hexane can you find?
Position isomerism In position isomerism, the basic carbon skeleton remains unchanged, but important groups are moved around on that skeleton. For example, there are two structural isomers with the molecular formula C 3H7Br. In one of them the bromine atom is on the end of the chain, whereas in the other it's attached in the middle. Task 3 Can you draw and name these two structural isomers?
If you made a model, there is no way that you could twist one molecule to turn it into the other one. You would have to break the bromine off the end and re-attach it in the middle. At the same time, you would have to move a hydrogen from the middle to the end.
Another similar example occurs in alcohols such as C4H9OH. Task 4 Can you name and draw two straight-chain structural isomers with this formula?
These are the only two possibilities provided you keep to a four carbon chain, but there is no reason why you should do that. You can easily have a mixture of chain isomerism and position isomerism - you aren't restricted to one or the other. Task 5
Can you draw two more alcohols with the molecular formula C4H9OH?
You can also get position isomers on benzene rings. Consider the molecular formula
C7H8Cl. There are four different isomers you could make depending on the position of the chlorine atom. In one case it is attached to the side-group carbon atom, and then there are three other possible positions it could have around the ring - next to the CH3 group, next-but-one to the CH3 group, or opposite the CH3 group. Functional group isomerism In this variety of structural isomerism, the isomers contain different functional groups - that is, they belong to different families of compounds (different homologous series).
For example, a molecular formula C3H6O could be either propanal (an aldehyde) or propanone (a ketone).
There are other possibilities as well for this same molecular formula - for example, you could have a carbon-carbon double bond (an alkene) and an -OH group (an alcohol) in the same molecule.
Another common example is illustrated by the molecular formula C3H6O2. Amongst the several structural isomers of this are propanoic acid (a carboxylic acid) and methyl ethanoate (an ester).
Task 6
Can you complete the activity sheet ‘ DF4.1 Modelling and naming alkanes’. References A-level Chemistry pages 280-281 Chemistry in Context pages 391-392
Learning Objectives Candidates should be able to: describe structural isomerism deduce the possible isomers for an organic molecule of known molecular formula.
Part 3: Combustion of alkanes
Task 1
Can you solve the anagrams to find the correct words in bold?
Alasken contain only carbon-carbon and carbon-hydrogen bonds: these are fairly strong and plan on nor. As a result alkanes are unaffected by polar reagents such as acids and alkalis, clues pinhole and police shelter. Indeed they were once known as the fans rap if, from the Latin words parum (little) and affinitas (affinity).
However, the few reactions, which they do undergo, are of great importance.
The alkanes are used as fuels. They are kinetically bleats in the presence of oxygen, but are energetically unstable with respect to their oxidation products. Given the necessary vacation it energy, they burn readily in air or oxygen. These reactions are highly cheer mix to. The reaction has a free-radical mechanism, and it occurs rapidly in the gas phase. Because it is a gas-phase acne riot, liquid and solid alkanes must first be vaporised. That is why less volatile alkanes burn less readily.
In the presence of a plentiful supply of oxygen, complete cubism onto occurs. The only products are carbon dioxide and water.
Task 2
Write balanced equations for the complete combustion of methane and hexane.
DH = -890 kJmol-1
DH = -4195 kJmol-1
As the number of carbon atoms increase, what trends do you observe?
Often the flame is yellow and luminous. Insufficient oxygen is available, leading to incomplete combustion. If you hold a beaker over this flame it will become sooty. Water is being formed together with carbon monoxide and carbon.
Task 3
Write balanced equations for the combustion of methane gas to carbon dioxide, carbon monoxide and carbon.
Carbon monoxide is a toxic gas. It binds irreversibly to the haemoglobin in your red blood cells preventing the uptake of oxygen.
Hydrocarbons contain sulphur impurities (sulphur atoms are found in protein molecules). These burn in air to produce sulphur dioxide (and, to a lesser extent, sulphur trioxide). This is thought to be the major cause of acid rain.
Internal combustion engines
Carbon monoxide is one of a number of pollutants formed by the incomplete combustion of petrol vapour in a car engine. Task 4
Use pages 411-413 of ‘Chemistry in Context’ to complete the table below.
Emission Source Chemical equation (where Problems associated with appropriate) this emission
CO2
CO
CxHy
NO
NOx
SO2
Catalytic converters
These devices help to remove CO, NOx and CxHx from car exhausts. They are made from a honeycomb of ceramic material onto which is spread a thin layer of metals such as Pt, Rh and Pd. These metals catalyse reactions between the pollutants and help to remove up to 90% of the harmful gases.
Task 5 Can you complete the equation below? This shows one of the reactions which take place in a catalytic converter.
CO + NO ®
CO and CxHx are oxidised by the air. Overall, the pollutant gases – CO and NOx and unburned hydrocarbons – are replaced by CO2, N2 and H2O, which are harmless! The catalytic reactions do not start working until the catalyst has reached a temperature of about 200 oC, so they are not effective until the engine has warmed up.
References A-level Chemistry pages 295 and 314-315 Chemistry in Context pages 411-413
Learning Objectives Candidates should be: aware of the general unreactivity of alkanes, including towards polar reagents; able to describe the chemistry of alkanes as exemplified by the combustion of ethane; able to describe and explain how the combustion reactions of alkanes lead to their use as fuels in industry, in the home and transport; able to recognize the environmental consequences of carbon monoxide, oxides of nitrogen and unburnt hydrocarbons arising from the internal combustion engine and of their catalytic removal. able to recognize the environmental consequences of gases that contribute to the enhanced greenhouse effect. Part 4: Chlorination
Alkanes such as methane do not react with chlorine (or bromine) at room temperature or in the dark. In the presence of sunlight (particularly ultraviolet light) or at high temperature, an explosive reaction occurs, producing chloromethane and hydrogen chloride (as well as other chlorinated methanes). Because the reaction requires ultraviolet light, it is called a photochemical reaction.
Task 1
Write a balanced equation for the reaction mentioned above.
This is an example of a free-radical substitution reaction which occurs in several stages. All of these stages together form the mechanism of the reaction. i) Initiation step
Cl2 ® 2Cl·
Ultraviolet light consists of very high-energy radiation. This supplies the energy needed to split the covalent bond in a chlorine molecule, forming atoms. This is known as homolytic fission because each free-radical retains one electron from each covalent bond. This process occurs first because the Cl-Cl bond in chlorine is weaker than the C-H bond in methane. ii) Propagation steps
The chlorine atoms, being free radicals, are highly reactive. When they collide with a methane molecule they combine with one of its hydrogen atoms, forming a new free radical: Using curly arrows, we can show the movement of the single electrons in this reaction:
The CH3· free radical can then react with another chlorine molecule:
and so the process continues. These two reactions (and other possible combinations) enable a chain reaction to occur; they are propagation steps. Each step is exothermic, so the chain reaction may be explosive. iii) Termination steps
The chain reaction ends when two free radicals combine to form a stable molecule. Possible termination steps include:
Such termination steps can lead to trace amounts of impurities, such as ethane, in the final product.
Further substitution
The reaction of a chlorine radical with methane extracts a hydrogen radical to form HCl, as in the first propagation stop above. This forms chloromethane which may also be attacked by a chlorine radical in a pair of propagation steps to form dichloromethane, and so on….. The proportion of different products formed depends on the proportions of chlorine and methane used.
Practically all the reactions of alkanes proceed by free-radical mechanisms, characterised by high activation energies and a tendency to proceed rapidly in the gas phase.
Task 2
Write the structural formulas of all the products you would expect to be formed when ethane reacts with excess chlorine in sunlight.
References A-level Chemistry pages 280-281 Chemistry in Context pages 391-392
Learning Objectives Candidates should be able to: describe the mechanism of free-radical substitution at methyl groups with particular reference to the initiation, propagation and termination reactions. describe the substitution of alkanes by chlorine and bromine. Part 5: Cracking
The composition of crude oil varies from place to place. In general, however, the amount of each fraction produced by distillation does not match the demand:
Approximate % Fraction Crude oil Demand Gases 2 4 Petrol and naphtha 16 27 Kerosene 13 8 Gas oil 19 23 Residue 50 38
It can be seen that in general there is a higher-demand for the lighter fractions and a surplus of heavy fractions. This imbalance is solved using a process known as cracking. Cracking is the name given to the breaking up of large hydrocarbon molecules into smaller and more useful bits.
Large alkanes are cracked to produce smaller alkanes, alkenes and sometimes even hydrogen. As it involves the breaking of carbon-carbon and carbon-hydrogen bonds it requires a large input of thermal energy or the use of a catalyst.
Cracking always produces many different products, and these can be separated in a fractionating column. Task 1
Write one possible equation for the cracking of C15H32.
What conclusions can you draw about cracking reactions?
......
...... Thermal cracking
Produces a high proportion of alkenes Temperatures range from 400-900oC Pressures up to 7000kPa
As the temperature increases, the cracking shifts from the middle of the chain towards the ends. To avoid complete decomposition to elements, the exposure (or residence) time is kept short (around 1 second).
Thermal cracking is initiated by the homolytic fission of a C-C bond to form two alkyl radicals. (Note C-C bonds are weaker than C-H bonds.)
Radicals are highly reactive atoms, or groups of atoms, with unpaired electrons. They are represented by the presence of a dot (e.g. Cl·). They are usually formed during the cleavage of non-polar covalent bonds.
The alkyl radical can then react in a number of ways. It could abstract a hydrogen atom from an alkane molecule to produce a different alkyl radical and a shorter alkane:
Or it could undergo further bond cleavage to form an alkene and a shorter alkyl radical:
In addition dehydrogenation, isomerisation and cyclisation reactions are also occurring. The alkenes produced, with their reactive double bonds, are used by the petrochemical industry as building blocks for larger organic molecules.
Catalytic cracking Produces a large proportion of branched alkanes, cycloalkanes and aromatic hydrocarbons Uses zeolite (crystalline aluminosilicate) catalysts Temperature around 450oC Pressure just above atmospheric Proportion of alkenes is small. This method is used mainly for the production of high-octane motor fuels. These branched alkanes prevent auto-ignition or ‘knocking’.
Mechanism proceeds via formation of carbocations (heterolytic fission). In the catalytic cracker the hot, vaporised oil fraction and the catalyst behave as a fluid. Some of the hydrocarbon mixture is broken down to carbon, which blocks the pores of the catalyst. This is burnt off in air at a high temperature, allowing the catalyst to be recycled.
Task 2
When an alkane is cracked, each molecule forms at least two new molecules. a) What reaction conditions are needed to cause cracking reactions in alkanes? b) Which of the following rules are true in writing an equation for cracking? i) There are more total molecules on the reactant side. ii) There are more total molecules on the product side. iii) All the crackate molecules are unsaturated. iv) Some of the crackate molecules are unsaturated. v) Crackate molecules are always smaller.
Task 3 – Complete the question sheet ‘Alkanes’.
References A-level Chemistry pages 310-311 Chemistry in Context pages 410-411
Learning Objectives Candidates should be able to suggest how ‘cracking’ can be used to obtain more useful alkanes and alkenes of lower Mr from larger hydrocarbon molecules.