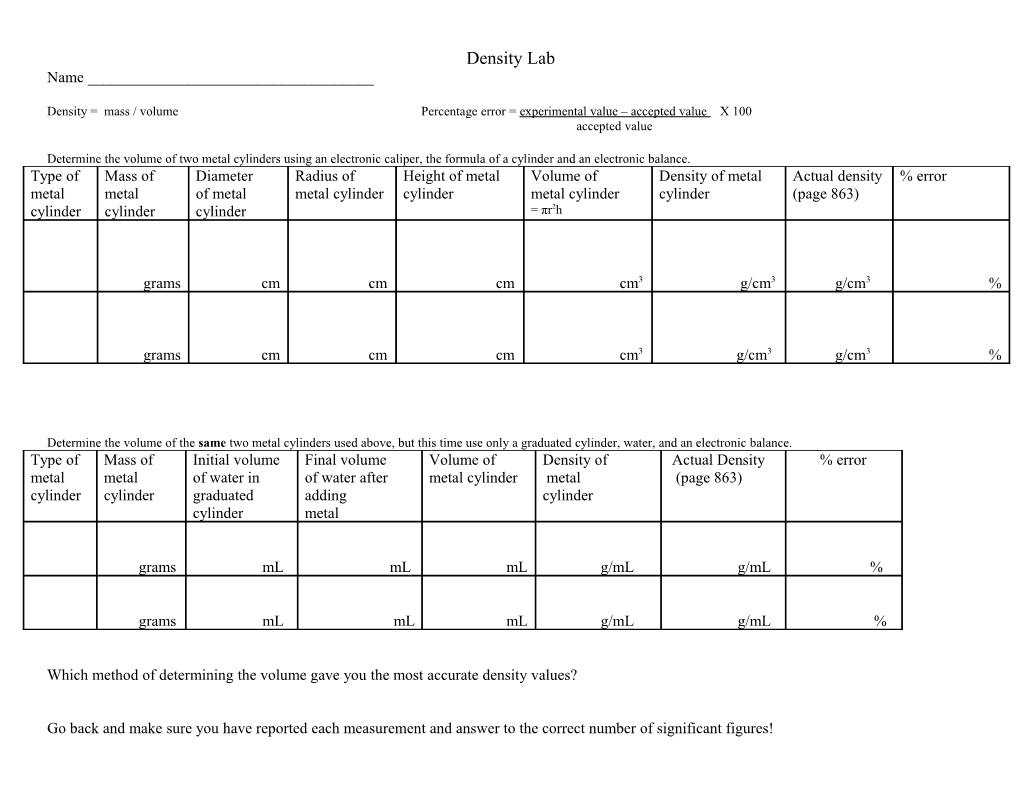
Density Lab Name ______
Density = mass / volume Percentage error = experimental value – accepted value X 100 accepted value
Determine the volume of two metal cylinders using an electronic caliper, the formula of a cylinder and an electronic balance. Type of Mass of Diameter Radius of Height of metal Volume of Density of metal Actual density % error metal metal of metal metal cylinder cylinder metal cylinder cylinder (page 863) cylinder cylinder cylinder = πr2h
grams cm cm cm cm3 g/cm3 g/cm3 %
grams cm cm cm cm3 g/cm3 g/cm3 %
Determine the volume of the same two metal cylinders used above, but this time use only a graduated cylinder, water, and an electronic balance. Type of Mass of Initial volume Final volume Volume of Density of Actual Density % error metal metal of water in of water after metal cylinder metal (page 863) cylinder cylinder graduated adding cylinder cylinder metal
grams mL mL mL g/mL g/mL %
grams mL mL mL g/mL g/mL %
Which method of determining the volume gave you the most accurate density values?
Go back and make sure you have reported each measurement and answer to the correct number of significant figures! Name ______
Determine the density of the colored liquids using only an electronic balance and a graduated cylinder. Color of liquid Mass of liquid Volume of liquid Density of liquid Will this liquid sink or float in pure water?
grams mL g/mL
grams mL g/mL
Use a caliper and a balance to find the density of a metal cube. Mass of metal cube Length of one side Volume of cube Density of cube What metal is it Actual density % error most likely (page 863) made of? (page 863)
grams cm cm3
Determine the density of an irregular-shaped object that has a string attached using water displacement. Shape and Mass of object Initial volume Final Volume of object Density of Identity of Actual density % error color of object of water volume (using water the object object (page 863) in graduated after displacement) cylinder adding object
grams mL mL mL g/mL g/mL %