Intro to Molecular Geometry
Let’s start by reviewing covalent naming. In your notebooks, write down the name for CO2 and BF3 and the formula for dihydrogen monoxide and nitrogen trihydride.
If you had to separate these four in to two groups, how could you pair them and why? CO2 and H2O have 3 atoms and BF3 and NH3 have 4
Let’s look at CO2 and H2O first. Let’s draw the electron-dot diagrams for them.
O C O H O H
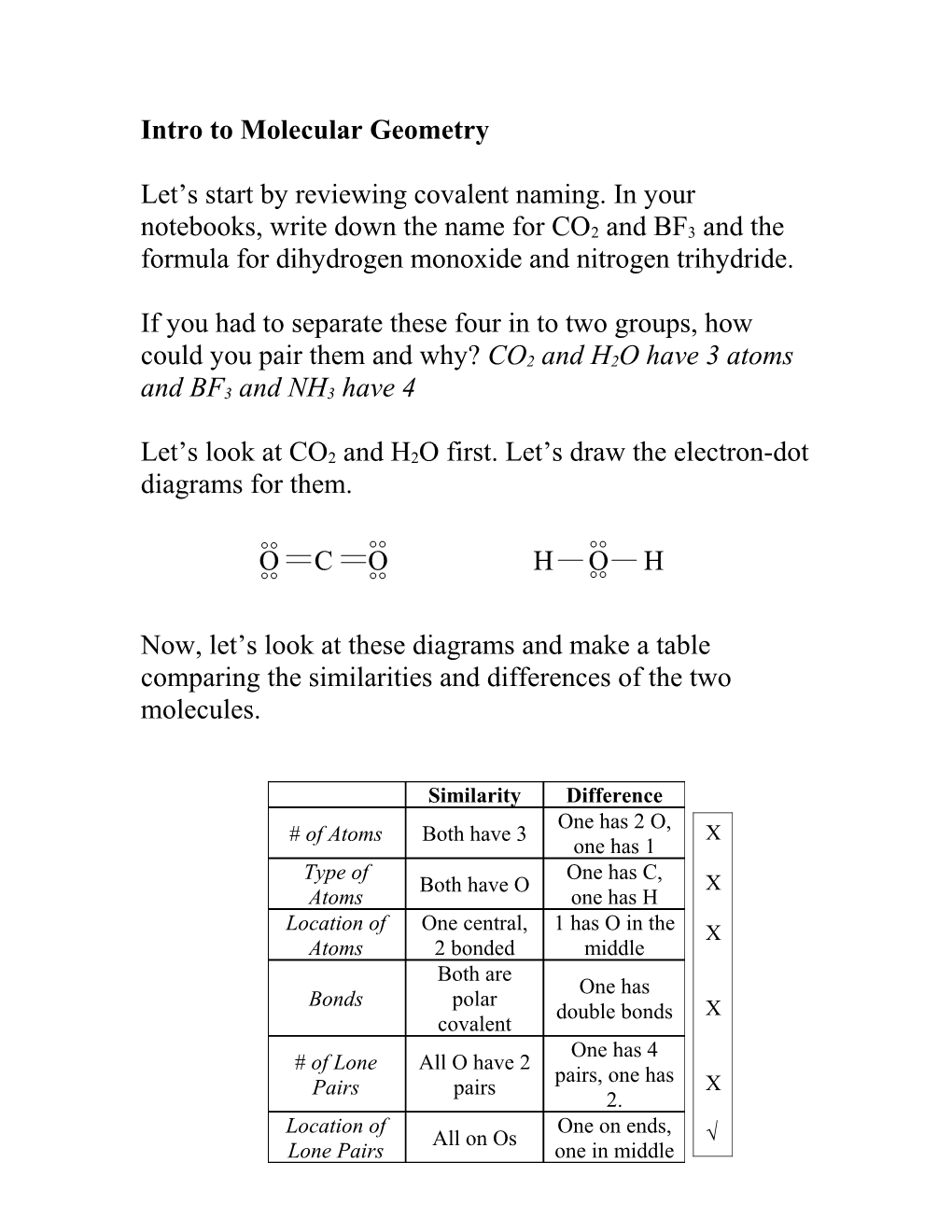
Now, let’s look at these diagrams and make a table comparing the similarities and differences of the two molecules.
Similarity Difference One has 2 O, # of Atoms Both have 3 X one has 1
Type of One has C, Both have O X Atoms one has H Location of One central, 1 has O in the X Atoms 2 bonded middle Both are One has Bonds polar double bonds X covalent One has 4 # of Lone All O have 2 pairs, one has Pairs pairs X 2. Location of One on ends, All on Os √ Lone Pairs one in middle Let’s build these two molecules using the model kits on your desks and compare their three dimensional structures now.
How can you describe the shapes of these molecules both qualitatively and quantitatively? CO2 is linear, 180 degrees, and H20 is bent, 105 degrees
Any ideas as to why two molecules that both have 3 atoms in them can look so different? The double bonding, or the location of the oxygens or electrons, etc.
How can we test that? Let’s build some other ternary molecules – nitrite, dihydrogen monocarbide, beryllium dihydride, and dinitrogen monoxide.
O N O H C H
H Be H N O N
Let’s see – is CO2 linear because it has double bonds? N2O is linear, but NO2 has a double bond and it’s not linear.
Okay, so we’ve ruled that out. Is CO2 linear because it has lone pairs on the oxygens on the ends? No, BeH2 is linear and it doesn’t have any lone pairs, and NO2 is bent and it has lone pairs.
Is H2O bent because it has less lone pairs? No – NO2 is bent and it has 6 lone pairs. So what does that leave us with? The location of the lone pairs.
Water, dihydrogen carbide, and nitrogen dioxide are bent because they have lone pairs on the central atom. Carbon dioxide, beryllium dihydride, and dinitrogen oxide are linear because they don’t.
We have determined this through a process of elimination, but let’s talk about why this happens. We know that electrons like to be in pairs, but do you think that these pairs would repel or attract each other? They would repel because they’re both negative.
When the pairs of electrons repel each other, the whole molecule becomes bent so that all of the electron pairs can be as far apart as possible. Next we will talk more about how this works in terms of the positions of the electrons and characterizing larger molecules.