The Chemistry of Dyes and Staining
Histological staining involves the use of dyes to highlight specific intra- or extracellular elements within tissue. A vast array of dyes and associated staining protocols exist in use. Each dye is targeted toward different cellular structures. The response to a given protocol can vary among samples. Many protocols are up to 100 years old, and were developed using partially characterized textile dyes. As a result, the detailed mechanism underlying many popular staining techniques is unclear. The Chemistry of Dyes
The human eye responds to wavelengths of light between 400 and 700 nanometers (the visible spectrum). The presence of all wavelengths in this spectrum is perceived as white light. The presence of one wavelength alone is seen as a color: Blue for 450 nm light, Red for 600 nm light, etc. Furthermore, if one color (wavelength) is removed from the full visible spectrum, the light is perceived as having the 'complementary color'. For example, materials which absorb at 450 nm (blue light) will appear carmine. In general, dyes appear colored because they absorb a particular wavelength in the visible region. The eye senses the reflected light as the complementary color.
The colors of the visible spectrum are represented above as three complementary pairs. The absorption of yellow light by the dye eosin produces a complementary purple color. Why Dyes Produce Color.
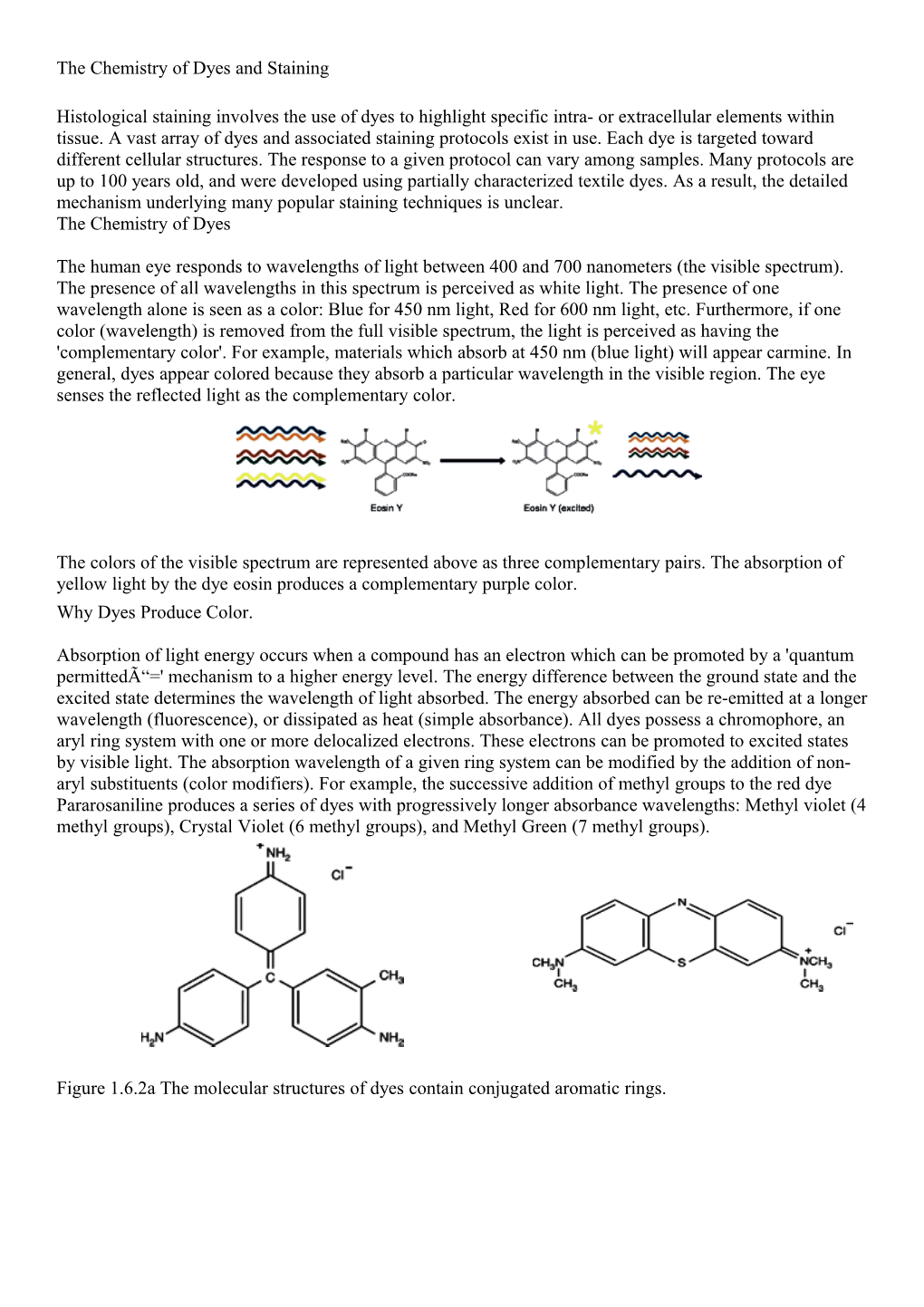
Absorption of light energy occurs when a compound has an electron which can be promoted by a 'quantum permittedÓ=' mechanism to a higher energy level. The energy difference between the ground state and the excited state determines the wavelength of light absorbed. The energy absorbed can be re-emitted at a longer wavelength (fluorescence), or dissipated as heat (simple absorbance). All dyes possess a chromophore, an aryl ring system with one or more delocalized electrons. These electrons can be promoted to excited states by visible light. The absorption wavelength of a given ring system can be modified by the addition of non- aryl substituents (color modifiers). For example, the successive addition of methyl groups to the red dye Pararosaniline produces a series of dyes with progressively longer absorbance wavelengths: Methyl violet (4 methyl groups), Crystal Violet (6 methyl groups), and Methyl Green (7 methyl groups).
Figure 1.6.2a The molecular structures of dyes contain conjugated aromatic rings. Figure 1.6.2b Simple absorption vs. fluorescence. The Chemistry of Staining
Staining procedures provide conditions which promote the binding of a given dye to specific cellular organelles or extracellular features. The utility of a staining procedure lies in its ability to bind dye only to selected structures, highlighting these structures in contrast with the rest of the section. To accomplish this, each procedure makes use of a subset of possible interactions between the dye and the cellular components. The major classes of interaction (bonds) are ionic, covalent, and hydrophobic. Ionic bonding results from the attraction between positive and negative charges. In solution, acidic groups (carboxylic or sulfonic acids, etc.) will lose a proton and become negatively charged (anionic). Basic groups (generally amines) will accept a proton to become positively charged cations. The pH of the solution determines the extent to which any chemical group is protonated or deprotonated, and a dye or biological molecule may have many such groups on its surface. Thus, altering the pH of a staining solution will alter the charges on the dye and the tissue molecules, and therefore alter the staining pattern. Ionic bonds are the predominant mode of interaction between tissues and dyes. Covalent bonding occurs between uncharged atoms that require the gain or loss of electrons to reach a stable configuration. In the usual scenario, the atoms involved donate electrons to a shared orbital. The atoms then share the electrons involved, and are bonded by the resulting orbital. Coordinate bonds are a subclass of covalent bonds in which one of the atoms donates all the electrons (two of them) which are then shared by both of the atoms participating in the bond. Except with mordants, covalent bonds are of little importance in staining. The presence of nonpolar molecules in an aqueous environment forces water molecules to assume a highly ordered arrangement, which is entropically disfavored. A large sphere of nonpolar molecules presents less surface area to the water than many dispersed molecules, so nonpolar materials tend to aggregate into their own phase. This sort of behavior, where molecules partition out of an aqueous solution, is called hydrophobicity, and the tendency of hydrophobic molecules to self associate is called the hydrophobic interaction. This phenomenon is utilized in staining lipids, which are hydrophobic. Hydrophobic stains will tend to dissolve into lipid rich regions of the section, highlighting them for analysis. Many dyes have a poor affinity for tissue when used alone. Various compounds, most often metal salts, have been found to enhance the staining of these dyes. These enhancing compounds are called mordants. The mechanism of action of the mordants is not clear, but it presumably involves coordination bonding between the metal and the dye, and then further coordination between this complex and the tissue.
NEXT TOPIC: Staining Procedures
Products Related to this Discussion: Alcian Blue Used in conjunction with PAS to differentiate acid and neutral mucopolysaccharides. Amido Black Useful forensic stain typically used for enhancing latent prints contaminated with blood. Basic Fuchsin Main ingredient of Schiff's reagent, a pH indicator, and stain for glycoproteins and mucopolysaccharide proteins. Biebrich Scarlet Plasma stain substituted for Acid Fuchsin in Masson's trichrome. Bromophenol Blue Commonly used tracking dye in electrophoresis. Fast Green FCF Recommended as a substitute for Light Green SF Yellowish in Masson's trichrome. Methyl Green In conjunction with Pyronin Y will differentiate RNA and DNA. Methylene Blue A sensitive stain specific for DNA and RNA. Pyronin Y In conjunction with Methyl Green will differentiate RNA and DNA