SUPPLEMENTAL INFORMATION
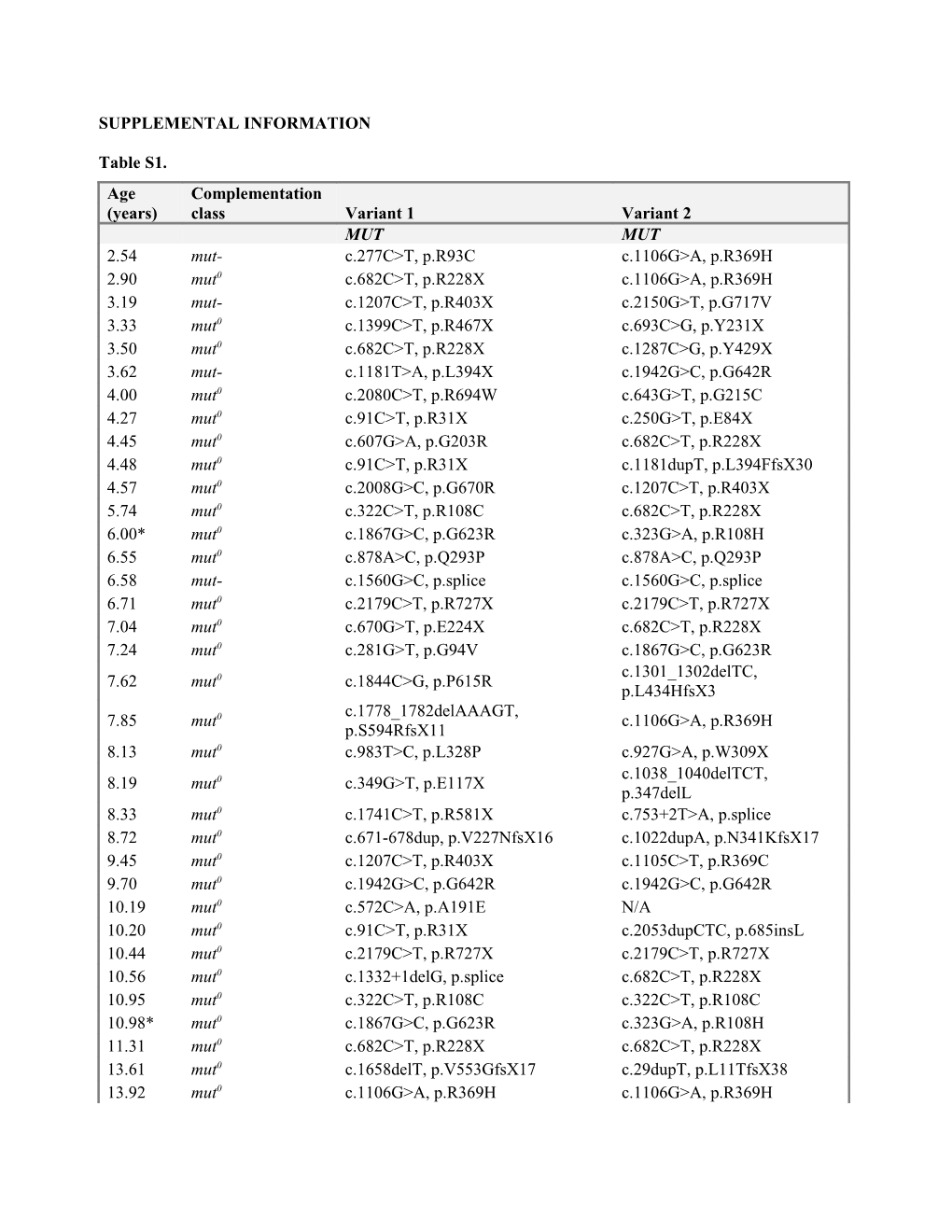
Table S1. Age Complementation (years) class Variant 1 Variant 2 MUT MUT 2.54 mut- c.277C>T, p.R93C c.1106G>A, p.R369H 2.90 mut0 c.682C>T, p.R228X c.1106G>A, p.R369H 3.19 mut- c.1207C>T, p.R403X c.2150G>T, p.G717V 3.33 mut0 c.1399C>T, p.R467X c.693C>G, p.Y231X 3.50 mut0 c.682C>T, p.R228X c.1287C>G, p.Y429X 3.62 mut- c.1181T>A, p.L394X c.1942G>C, p.G642R 4.00 mut0 c.2080C>T, p.R694W c.643G>T, p.G215C 4.27 mut0 c.91C>T, p.R31X c.250G>T, p.E84X 4.45 mut0 c.607G>A, p.G203R c.682C>T, p.R228X 4.48 mut0 c.91C>T, p.R31X c.1181dupT, p.L394FfsX30 4.57 mut0 c.2008G>C, p.G670R c.1207C>T, p.R403X 5.74 mut0 c.322C>T, p.R108C c.682C>T, p.R228X 6.00* mut0 c.1867G>C, p.G623R c.323G>A, p.R108H 6.55 mut0 c.878A>C, p.Q293P c.878A>C, p.Q293P 6.58 mut- c.1560G>C, p.splice c.1560G>C, p.splice 6.71 mut0 c.2179C>T, p.R727X c.2179C>T, p.R727X 7.04 mut0 c.670G>T, p.E224X c.682C>T, p.R228X 7.24 mut0 c.281G>T, p.G94V c.1867G>C, p.G623R c.1301_1302delTC, 7.62 mut0 c.1844C>G, p.P615R p.L434HfsX3 c.1778_1782delAAAGT, 7.85 mut0 c.1106G>A, p.R369H p.S594RfsX11 8.13 mut0 c.983T>C, p.L328P c.927G>A, p.W309X c.1038_1040delTCT, 8.19 mut0 c.349G>T, p.E117X p.347delL 8.33 mut0 c.1741C>T, p.R581X c.753+2T>A, p.splice 8.72 mut0 c.671-678dup, p.V227NfsX16 c.1022dupA, p.N341KfsX17 9.45 mut0 c.1207C>T, p.R403X c.1105C>T, p.R369C 9.70 mut0 c.1942G>C, p.G642R c.1942G>C, p.G642R 10.19 mut0 c.572C>A, p.A191E N/A 10.20 mut0 c.91C>T, p.R31X c.2053dupCTC, p.685insL 10.44 mut0 c.2179C>T, p.R727X c.2179C>T, p.R727X 10.56 mut0 c.1332+1delG, p.splice c.682C>T, p.R228X 10.95 mut0 c.322C>T, p.R108C c.322C>T, p.R108C 10.98* mut0 c.1867G>C, p.G623R c.323G>A, p.R108H 11.31 mut0 c.682C>T, p.R228X c.682C>T, p.R228X 13.61 mut0 c.1658delT, p.V553GfsX17 c.29dupT, p.L11TfsX38 13.92 mut0 c.1106G>A, p.R369H c.1106G>A, p.R369H 18.19 mut0 c.643G>T, p.G215C c.643G>T, p.G215C 18.51 mut0 c.572C>A, p.A191E c.655A>T, p.N219Y 24.21 mut0 c.1741C>T, p.R581X c.1741C>T, p.R581X 24.52 mut- c.2150G>T, p.G717V c.2150G>T, p.G717V 24.76 mut0 c.1106G>A, p.R369H c.1106G>A, p.R369H 24.77 mut0 c.1048C>T, p.H350Y c.655A>T, p.N219Y 24.88 mut0 c.572C>A, p.A191E c.682C>T, p.R228X 25.44 mut0 c.935G>T, p.G312V c.1909G>A, p.G637R 25.96 mut- c.1760A>C, p.Y587S c.2150G>T, p.G717V 26.41 mut- c.2053dupCTC, p.685insL c.2053dupCTC, p.685insL 32.71 mut0 c.826G>T, p.E276X c.1106G>A, p.R369H MMAA MMAA 2.79 cblA c.592_595delACTG, p.T198SfsX6 c.387C>A, p.Y129X 6.35 cblA c.433C>T, p.R145X c.1075C>T, p.R359X 10.42 cblA c.450dupG, p.P151AfsX19 c.433C>T, p.R145X 13.50 cblA c.349G>T, p.E117X c.64C>T, p.R22X 13.81 cblA c.433C>T, p.R145X c.433C>T, p.R145X 22.12 cblA c.450dupG, p.P151AfsX19 c.440G>A, p.E147G 33.32 cblA c.433C>T, p.R145X c.433C>T, p.R145X 33.36 cblA c.450dupG, p.P151AfsX19 c.450dupG, p.P151AfsX19 34.34 cblA c.433C>T, p.R145X c.433C>T, p.R145X MMAB MMAB 8.80 cblB c.556C>T, p.R186W c.556C>T, p.R186W 17.90 cblB c.291-1G>A, p.splice c.556C>T, p.R186W 18.95 cblB c.556C>T, p.R186W c.556C>T, p.R186W 21.17 cblB c.700C>T, p.Q234X N/A 23.21* cblB c.700C>T, p.Q234X c.556C>T, p.R186W 24.89* cblB c.700C>T, p.Q234X c.556C>T, p.R186W
Table S1. The isolated methylmalonic acidemia subtype based on 14C-propionate incorporation and complementation assays in fibroblast and the molecular genetic analysis of the MUT, MMAA and MMAB genes, respectively (GeneDx; Gaithersburg, MD) are provided for each patient listed by age and subtype. Asterisks indicate siblings with mut and cblB deficiency respectively. Table S2.
MMA/PA MMA/PA Standard Human Free AA Medical food Medical food Infant breast milk Infant formula Stage 1 Stage 2 formula Protein or Equivalent (g/100kcal) 1.8 2.7 – 3.1 5.1 – 13.1 2.1 2.8-3.0 Amino acids (mg/g protein) Leucine 101 92 - 154 92 - 162 102 - 106 122-123 Valine 56 0 0 60 - 63 78 – 87 Isoleucine 57 0 - 8 0 - 8 54 - 62 70 – 71 Methionine 16 0 0 20 - 26 19 – 28 Threonine 47 0-7 0-7 54 - 55 50 – 60
Table S2. Protein content and amino acid composition of special methylmalonic and propionic acidemia formulas (MMA/PA) compared to breast milk, regular infant formulas and free/elemental amino acid (AA)- based formulas*.
*Footnote: Formulas used for the creation of this table are listed below: Propimex1/2 (Abbott Laboratories, Columbus, OH), OA1/2 (Mead Johnson, Glenview, IL), XMTVI Analog, Maxamaid or Maxamum (Nutricia North America, Cedar Knolls, NJ) and MMA/PA Express (Vitaflo USA, Alexandria, VA). Standard Infant formulas: Enfamil Premium (Mead Johnson Nutrition, Glenview, IL), Similac Advance (Abbott Laboratories, Columbus, OH) Free amino acid-formulas: Elecare (Abbott Laboratories, Columbus, OH), Neocate Infant (Nutricia North America, Cedar Knolls, NJ) Composition of human milk was based on: National Research Council. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients).Washington, DC: The National Academies Press, 2005. Metabolic pro (https://metabolicpro.org) Supplemental Figure Legends:
Figure S1: The height, weight and BMI of each patient <20y of age are represented on the CDC growth charts (black circles: mut; blue circles: cblA; green circles: cblB; and red circles: transplanted patients). 12/18 males (66%) and 11/16 females (68%) with mut MMA had a height below the 10th centile and 5/18 males (27%) and 4/16 females (25%) had a BMI over the 85th centile, classified as overweight, while 1 female had a BMI over the 95th centile, which classified her as obese. Growth outcomes by age group (2-9y, 10-18y and over 18y old) were worse than the French patient cohort by Touati et al1. Height Z-score for patients in the younger age group in the current study was -1.76 ± 1.64 (N = 24), significantly lower that the mean of -0.47 ± 1.21 (N = 39) or -0.45 ± 1.38 (N = 29) presented by the French group for 3y and 6y of age respectively (P < 0.001 for both). Similarly the height Z-score of -2.63 ± 1.74 (N = 7) in the 10-18y old age group was significantly different than the -0.71 ± 1.87 (N = 15) presented for 11y of age in the French cohort (P = 0.033). It is intriguing that only 41-50% of isolated MMA patients in the French study received medical foods compared to 85% in our cohort. Furthermore, none of these patients received medical foods prior to age 3 years, and none consumed medical foods in excess of natural protein. Although the reasons behind the significantly different growth outcomes between our study and the French cohort are likely multifactorial, the vast difference in the approach towards medical foods may represent one of the variables.
Figure S2: Illustrative examples are provided to support the causal relationship between the medical food consumption and plasma amino acid effects. A. Morning and afternoon blood samples are presented from a single mut0 MMA patient during three different NIH hospitalizations. The early morning specimens were drawn after the completion of overnight feeds containing the majority of patient’s daily nutrients, including the special MMA/PA formula, and show higher concentrations of leucine compared to valine, isoleucine and methionine (µmol/L), in contrast to levels drawn later in the day after natural-protein-containing lunch and other snacks, when the ratios of BCAA in the plasma are closer to normal with Val>Leu>Ile plasma levels. B and C. Two siblings with cblB, aged 2y and 4y received a generous amount of natural protein daily, of 2.2 and 1.9g/kg/day respectively and in addition a modest amount of MMA/PA formula of about 0.9- 1.28g/k/d. Despite adequate protein intake, plasma amino acids analysis repeatedly showed very low levels of Val (26-72 µmol/L, normal range: 74-311 µmol/L) and Ile (2-20 µmol/L, normal range: 22-107 µmol/L). After the special formulas were completely withdrawn in an iso-caloric fashion, plasma AA levels returned to normal in both patients. Taken together, isolated valine and isoleucine deficiencies can be avoided or reversed by optimizing the ratio of complete vs. incomplete protein intake, rather than further increasing the complete protein prescribed or supplementing with the individual amino acids. If the patient consumes complete protein close to the RDA amount and plasma concentrations of valine and/or isoleucine, but not leucine, are decreased, replacing a portion of medical foods by a protein-free mixture/formula in an isocaloric fashion may be sufficient to normalize plasma amino acid concentrations. Given the immediate relation of plasma amino acid concentrations to the medical foods intake, blood for plasma amino acid quantitation should be drawn 2-4 hours after the last feeding. Medical foods should be used in moderation. While the ideal ratio to complete protein remains to be determined, in general, it should not exceed 1:1. At no time should patients with MMA require supplementation with valine or isoleucine.
Figure S3: A. Bivariate correlations are depicted for mut0 patients between protein intake (%RDA daily complete protein) and lean body mass (r=0.387, P=0.021, R2= 0.150); B and C. Strong correlations were observed between height Z-score and renal parameters, such as creatinine (r=-0.410, P=0.02, R2=0.221) and cystatin- C (r=-0.669, P=0.005, R2=0.448), while an abnormal renal function was associated with a significantly higher plasma MMA levels (r=0.785 and 0.831, respectively, P<0.001, R2=0.691); D. In addition, serum protein (r=0.451, P=0.007, R2=0.204) and albumin (r=0.608, P<0.001, R2=0.369) concentrations correlated with platelet counts. E. Renal function, represented as estimated GFR (eGFR), showed a strong negative correlation with serum prealbumin (transthyretin) concentrations (r=-0.659, P=0.001, R2=0.434). Serum protein or albumin concentrations were not affected by renal function. These findings render prealbumin a very poor biomarker of protein status in patients with MMA, who are prone to renal failure, in contrast to PA or other metabolic disorders.