CHM 123 HW – Chapter 13-Chemical Kinetics Name Due Tuesday 10/18/16 Don’t forget to show all your work
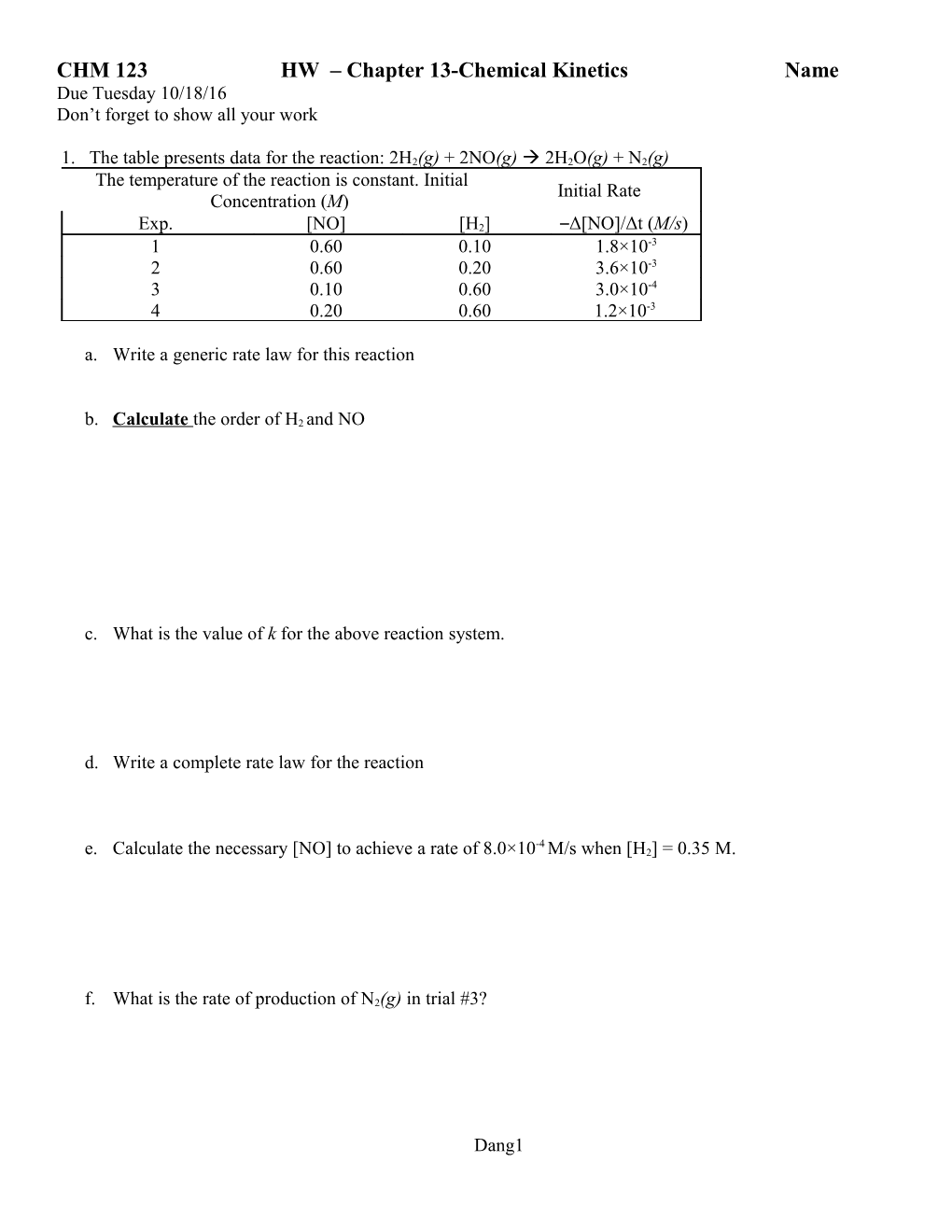
1. The table presents data for the reaction: 2H2(g) + 2NO(g) 2H2O(g) + N2(g) The temperature of the reaction is constant. Initial Initial Rate Concentration (M) Exp. [NO] [H2] –Δ[NO]/Δt (M/s) 1 0.60 0.10 1.8×10-3 2 0.60 0.20 3.6×10-3 3 0.10 0.60 3.0×10-4 4 0.20 0.60 1.2×10-3
a. Write a generic rate law for this reaction
b. Calculate the order of H2 and NO
c. What is the value of k for the above reaction system.
d. Write a complete rate law for the reaction
-4 e. Calculate the necessary [NO] to achieve a rate of 8.0×10 M/s when [H2] = 0.35 M.
f. What is the rate of production of N2(g) in trial #3?
Dang1 2. The first-order reaction, 2 N2O(g) → 2 N2(g) + O2(g), has a rate constant equal to 0.76 s-1 at 1000 K. How long will it take for the concentration of N2O to decrease to 42% of its initial concentration?
3. Nitrosyl bromide decomposes at 10.0oC 2 NOBr(g) 2 NO(g) + Br2(g) Use the following kinetic data to determine the order of the reaction and the value of the rate constant for the consumption of NOBr (hint: using graphical method and attach or draw it) Time(s) 0 10 20 30 40 [NOBr] 0.0400 0.0303 0.0244 0.0204 0.0175
4. Butadiene (C4H6) reacts with itself to form a dimer with the formula C8H12. The reaction is second order in -2 -1 -1 C4H6. IF the rate constant at a particular temperature is 4.0 x 10 M s and the initial concentration of
C4H6 is 0.0200M: a. What is its molarity after a reaction time of 1.00 h?
b. What is the time (in hours) when C4H6 concentration reaches a value of 0.0020M?
c. What is the half-life (in minutes?)
d. How many minutes does it take for the concentration of C4H6 to drop from 0.0100M to 0.0050M
Dang2