Tear off the top pages (pg. 1 - 3)It is Your reference tables and scratch paper Ka TABLE, ACID-IONIZATION CONSTANTS AT 25°C*
Substance Formula Ka HC H O Acetic acid5-5 2 3 2 1.7 X 10-53 HC H O Benzoic acid5-53 7 5 5 6.3 X 10-53 5 Boric acid5-53 H3BO3 5.9 X 10-103 5-53 H2CO3 -73 Carbonic acid 33 4.3 X 10 - -113 HCO3 4.8 X 10 Cyanic acid5 HOCN5 -43 3.5 X 10 33 5 5 3 Formic acid HCHO2 1.7 X 10-4 5 3 Hydrocyanic acid HCN 4.9 X 10-103
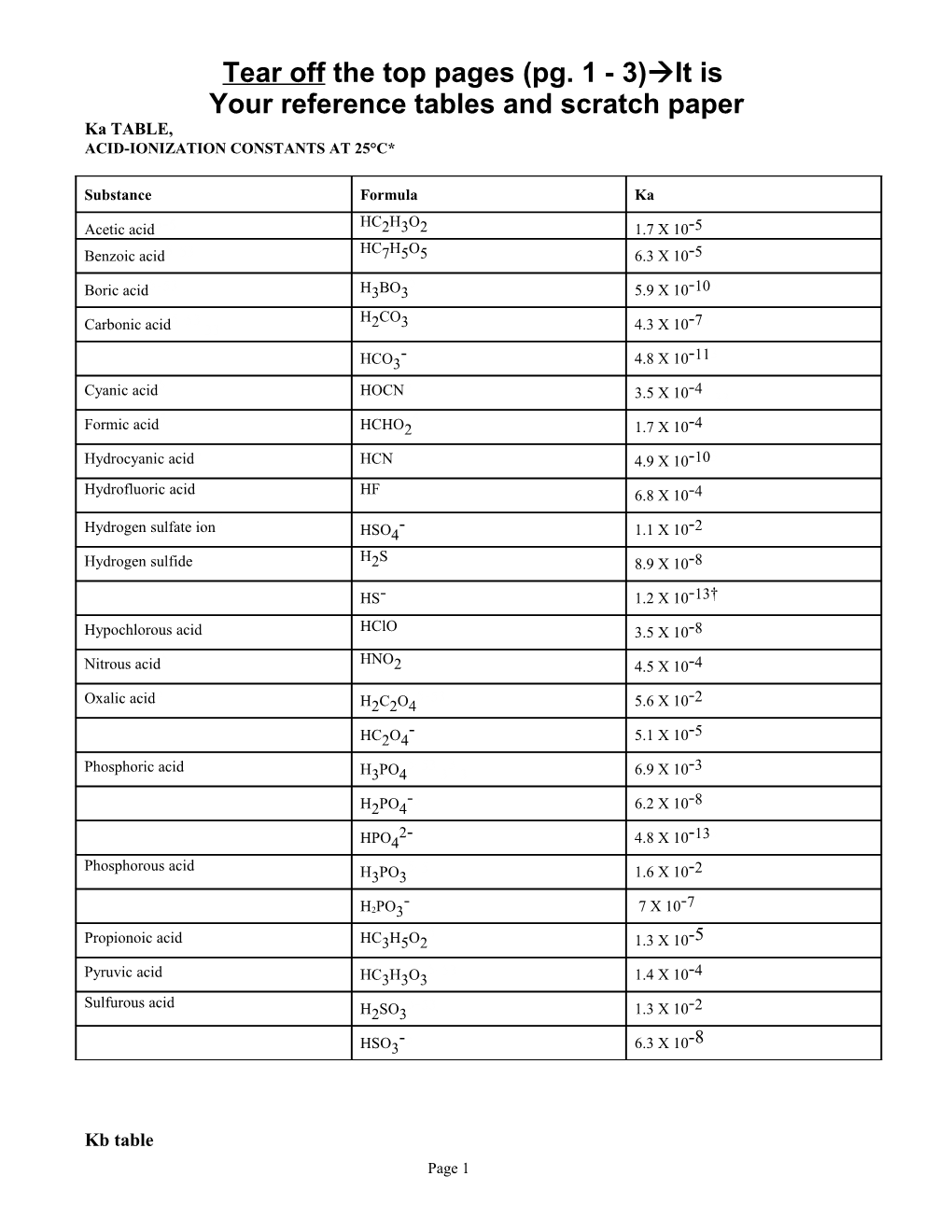
Hydrofluoric acid HF 6.8 X 10-4
Hydrogen sulfate ion5 - -23 HSO4 1.1 X 10 5 H S Hydrogen sulfide 2 8.9 X 10-83
HS- 1.2 X 10-13† 5 Hypochlorous acid HClO 3.5 X 10-83 5 HNO Nitrous acid 2 4.5 X 10-43
Oxalic acid5 5-53 -23 H2C2O4 5.6 X 10 - -53 HC2O4 5.1 X 10 Phosphoric acid5 5-53 3 -33 H3PO4 3 3 6.9 X 10 -5 -83 H2PO4 6.2 X 10 2- -133 HPO4 4.8 X 10 Phosphorous acid 5-5 -23 H3PO3 1.6 X 10 - -73 H2PO3 7 X 10 5 5 Propionoic acid HC3H5O2 1.3 X 10-53 Pyruvic acid5 5-53 -4 HC3H3O3 1.4 X 10 Sulfurous acid 5-53 -23 H2SO3 1.3 X 10 -5 -83 HSO3 6.3 X 10
Kb table Page 1 DISSOCIATION CONSTANTS FOR BASES Dissocaiation -5Base Formula Constants at 25°C -5 -5 -5 -5 -5 Ammonia 8 NH3 41.76 X 10 -5 -5 -5 -10 Anniline 8 C6H5NH2 43.94 X 10 -5 -5 -5 -4 1-Butylamine 8 CH3(CH2) 2CH2NH2 44.0 X 10 -5 -5 -5 -4 Dimethylamine 8 (CH3) 2NH 45.9 X 10 -5 -5 -5 -5 Ethanolamine 8 HOC2H4NH2 43.18 X 10 -5 -5 -5 -4 Ethylamine 8 CH3CH2NH2 44.28 X 10 -5 -5 -5 -5 Ethylenediamine NH2C2H4NH2 4K = 8.5 X 10 -5 -5 -8 88 4K = 7.1 X 10 -5Hydrazine-5 -5H NNH -6 8 2 2 41.3 X 10 -5Hydroxlamine-5 -5HONH -8 8 2 41.07 X 10 -5Methylamine-5 -5CH NH -4 8 3 2 44.8 X 10 -5Piperidine-5 -5C H N -3 8 5 11 41.3 X 10 -5Pyridine-5 -5C H N -9 8 5 5 41.7 X 10 -5Trimethyl amine-5 -5 (CH ) N -5 8 3 3 46.25 X 10
Page 2 CHEM. 111
CLS EXAM IV NAME______last first ______THERE ARE 5 PAGES TO THIS EXAM (including the cover page) Significant Figures must be correct. All set-ups must be shown (27 points) 1. Calculate the pH of the following: a. 0.500 M H 2CO3
ANSWER______3.33______
b. 0.0325 moles of NaHC2O4 is added to 100.0 mL of 2.00 M H2C2O4
ANSWER______0.46______a. 0.400 M potassium phosphate
ANSWER______12.96______Page 3 -26 (10 points) 2. Calculate the solubility of Ca3(PO4)2 in water (Ksp = 1 x 10 ) .
ANSWER______2 x 10-6______
(13 points) 3. Calculate the pH of a solution if 200.0 mL of .250M H2SO4 is added to 200.0 mL of 0.500 M methylamine.
ANSWER______5.64______
Page 4 (13 points) 4. Calculate the pH of the resulting solution when 35.00 mL of 0.200M NaOH is added to 70.00 mL of 0.200 M NaH2PO4
ANSWER______7.20______(13 points) 5. Calculate the number of moles of HCl needed to be added to 2.00 L of 0.2500M pyridine to make a buffer with a pH of 8.00.
ANSWER______8.48 x 10-4 moles HCl______
Page 5 (10 points) 6. 25.0 mL of 0.00100 M Na2SO4 is added to a solution containing 100.0 mL of 0.0020 M CaCl2 Will a precipitate form? You must show calculations to justify your answer. -5 Ksp of CaSO4 = 2.4 x 10
ANSWER______No ppt will form______(15 points) 7. Will the following solutions be acidic, basic or neutral? Write the equilibrium equations (and calculations, if necessary) to justify your answer.
a. NaHSO3
Acidic
b. Al(NO3)3 Acidic
c. CH3CH2NH3NO2
Acidic
Page 6