Mock Exam III CH115 Dr. Hamilton
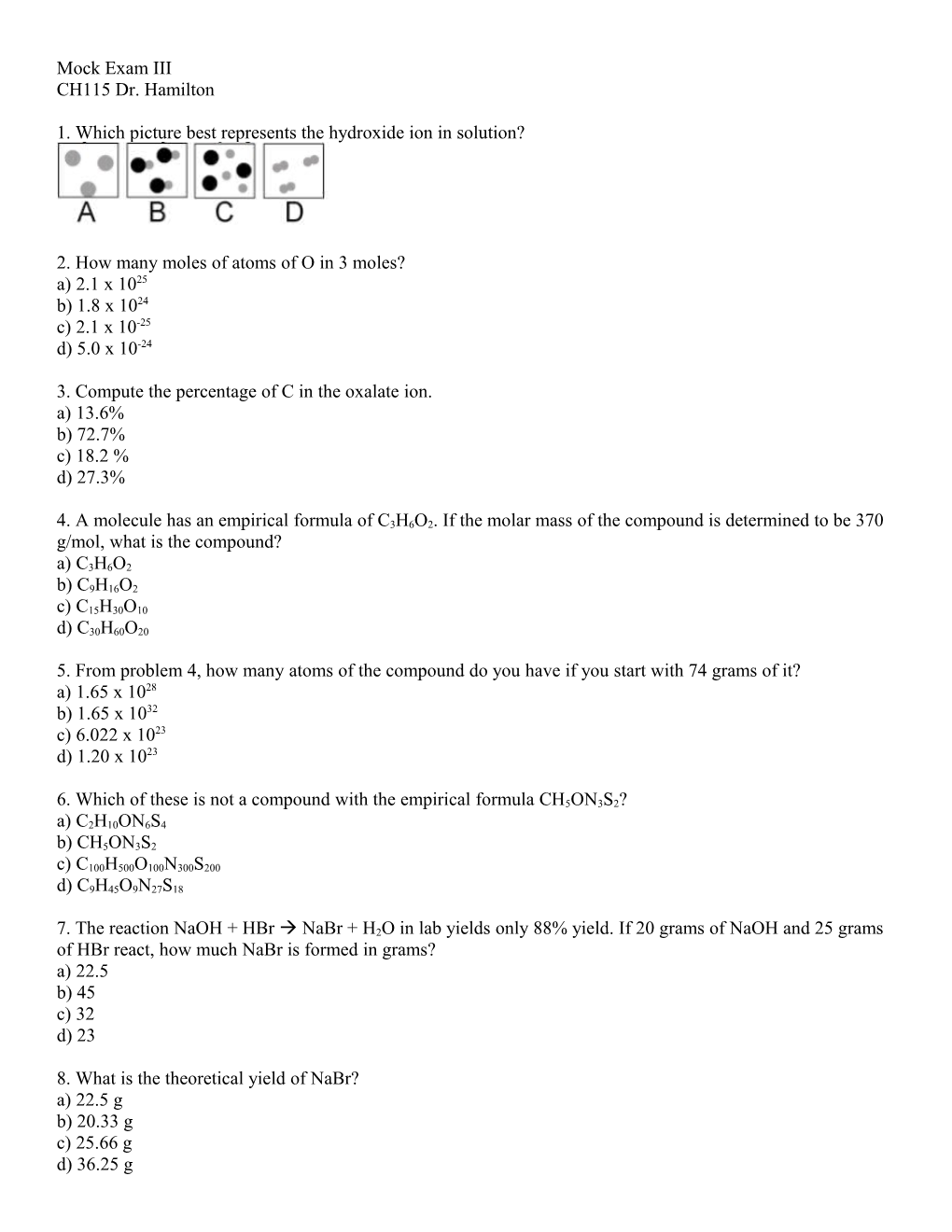
1. Which picture best represents the hydroxide ion in solution?
2. How many moles of atoms of O in 3 moles? a) 2.1 x 1025 b) 1.8 x 1024 c) 2.1 x 10-25 d) 5.0 x 10-24
3. Compute the percentage of C in the oxalate ion. a) 13.6% b) 72.7% c) 18.2 % d) 27.3%
4. A molecule has an empirical formula of C3H6O2. If the molar mass of the compound is determined to be 370 g/mol, what is the compound? a) C3H6O2 b) C9H16O2 c) C15H30O10 d) C30H60O20
5. From problem 4, how many atoms of the compound do you have if you start with 74 grams of it? a) 1.65 x 1028 b) 1.65 x 1032 c) 6.022 x 1023 d) 1.20 x 1023
6. Which of these is not a compound with the empirical formula CH5ON3S2? a) C2H10ON6S4 b) CH5ON3S2 c) C100H500O100N300S200 d) C9H45O9N27S18
7. The reaction NaOH + HBr NaBr + H2O in lab yields only 88% yield. If 20 grams of NaOH and 25 grams of HBr react, how much NaBr is formed in grams? a) 22.5 b) 45 c) 32 d) 23
8. What is the theoretical yield of NaBr? a) 22.5 g b) 20.33 g c) 25.66 g d) 36.25 g 9. In principle one could use only the non-global warming part of natural gas, H2, via CH4 (g) → C (s) + 2 H2 (g).What classification of reaction is this? a) decomposition b) combustion c) single displacement d) double displacement
10. What is the oxidation number oxygen in KO2? a) 0 b) -1/2 c) -1 d) -2
11. In the reaction between calcium hydroxide and barium sulfate, what is the precipitate formed? a) calcium ions b) hydroxide ions c) calcium sulfate d) barium hydroxide
12. What is the oxidation number of phosphorus in H3PO4? a) -1 b) 1 c) 3 d) 5
13. What are the spectator ions in this unbalanced equation? KOH + H2SO4 K2SO4 + H2O. a) water b) OH-, H+ + 2- c) K , SO4 d) K+, H+
14. What is the balanced equation from left to right in the equation above. a) 1, 2, 1, 2 b) 2, 1, 2, 1 c) 2, 1, 1, 2 d) 1, 2, 2, 1
15. In the combustion of 2-methylbutane, 10 grams of water is formed. How much CO2 is formed? a) 10 g b) 15 g c) 20 g d) 25 g Use this table to answer questions 16-18.
16. Will the reaction occur? If yes, how many electrons are transferred? Co + Mg2+ Co2+ + Mg. a) no b) yes, 1 c) yes, 2 d) yes, 3
17. Will this reaction occur? If yes, how many electrons are transferred? Mn + 2H2O(g) Mn(OH)2 + H2(g) a) no b) yes, 1 c) yes, 2 d) yes, 3
18. Will this reaction occur? What molecule is reduced? Ni + H2SO4 NiSO4 + H2(g) a) no b) yes, Ni c) yes, H2SO4 d) yes, H2
- 19. How many resonance structures does NO3 have? a) 4 b) 3 c) 2 d) 0
20. In this reaction, what is the reducing agent? Fe2O3 + 3CO 2Fe + 3CO2 a) Fe2O3 b) CO c) Fe d) CO2 Short Answer
1. Balance these equations (include states of matter for part a and d). a)
b)
c)
d)
2. Look at part 1c. Assign oxidation numbers to each atom and then label which is the reducing agent and which is the oxidizing agent.
3. In the combustion of 20 grams of octene with 15 grams of oxygen, 4 grams of carbon dioxide is produced. What is the percent yield and how many atoms of excess reagent is left over after the reaction goes through completion?