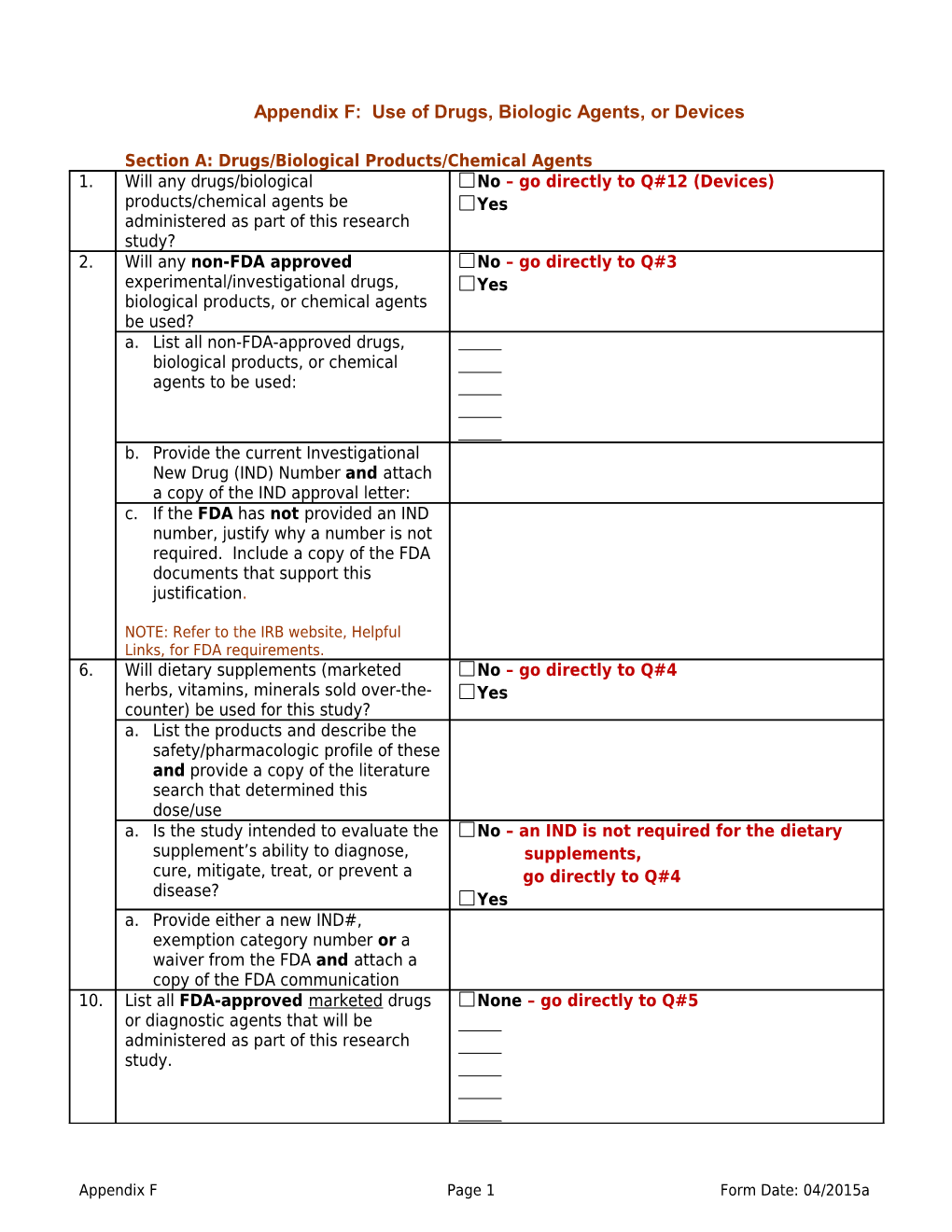
Appendix F: Use of Drugs, Biologic Agents, or Devices
Section A: Drugs/Biological Products/Chemical Agents 1. Will any drugs/biological No – go directly to Q#12 (Devices) products/chemical agents be Yes administered as part of this research study? 2. Will any non-FDA approved No – go directly to Q#3 experimental/investigational drugs, Yes biological products, or chemical agents be used? a. List all non-FDA-approved drugs, biological products, or chemical agents to be used:
b. Provide the current Investigational New Drug (IND) Number and attach a copy of the IND approval letter: c. If the FDA has not provided an IND number, justify why a number is not required. Include a copy of the FDA documents that support this justification.
NOTE: Refer to the IRB website, Helpful Links, for FDA requirements. 6. Will dietary supplements (marketed No – go directly to Q#4 herbs, vitamins, minerals sold over-the- Yes counter) be used for this study? a. List the products and describe the safety/pharmacologic profile of these and provide a copy of the literature search that determined this dose/use a. Is the study intended to evaluate the No – an IND is not required for the dietary supplement’s ability to diagnose, supplements, cure, mitigate, treat, or prevent a go directly to Q#4 disease? Yes a. Provide either a new IND#, exemption category number or a waiver from the FDA and attach a copy of the FDA communication 10. List all FDA-approved marketed drugs None – go directly to Q#5 or diagnostic agents that will be administered as part of this research study.
Appendix F Page 1 Form Date: 04/2015a Appendix F: Use of Drugs, Biologic Agents, or Devices
a. Will these drugs/agents be used for No – an IND is not required for the FDA- a new indication? (e.g., new use, approved new combination of two or more drugs/agents, go directly to Q#5 drugs, altered dose, new route of Yes administration, new participant population, etc). b. If this is a new indication, describe the planned use of the study drug/biologic product/chemical agent c. If this is a new indication, does the study meet all of the No following criteria? Yes The drug product is lawfully marketed in the United States. The investigation is not intended to be reported to FDA as a well-controlled study in support of a new indication and there is no intent to use it to support any other significant change in the labeling of the drug. In the case of a prescription drug, the investigation is not intended to support a significant change in the advertising for the drug. The investigation does not involve a route of administration, dose, patient population, or other factor that significantly increases the risk (or decreases the acceptability of the risk) associated with the use of the drug product (21 CFR 312.2(b) (1)(iii)). The investigation is conducted in compliance with the requirements for review by an IRB (21 CFR part 56) and with the requirements for informed consent (21 CFR part 50). The investigation is conducted in compliance with the requirements of § 312.7 (i.e., the investigation is not intended to promote or commercialize the drug product). a. Provide either a new IND#, exemption category number or a waiver from the FDA and attach a copy of the FDA communication:
15. Has a copy of an investigational drug No, state why not: brochure and/or package insert been Yes included with this submission? 16. Will this study involve a placebo? No – go directly to Q# 7 Yes a. Select the box that best describes With standard therapy or active treatment. the reason for placebo use. In place of standard therapy or active treatment. If current medication is stopped, explain how participants safety will be monitored: Other (describe): 18. Will the drugs, supplements, agents, or No placebo be administered under the Yes – go directly to Q# 8 direct supervision of the Principal Investigator or a co-investigator responsible to the PI?
Appendix F Page 2 Form Date: 04/2015a Appendix F: Use of Drugs, Biologic Agents, or Devices
a. State who will administer:
19. Describe the drug accountability plan that includes receiving, storing, dispensing, and final disposition and accountability of the drug. 20. Will the study involve use of infectious agents or non-plasmid No recombinant DNA? Yes (SEE NOTE)
NOTE: Use of certain biological agents requires review and approval by the WSU Institutional Biosafety Committee (IBC). To determine if this applies to your research, please contact the WSU Biosafety Officer at (313) 577-1200. 10. Will the study involve use of plasmid recombinant DNA? No Yes (SEE NOTE) NOTE: Use of certain biological agents requires review and approval by the WSU Institutional Biosafety Committee (IBC). To determine if this applies to your research, please contact the WSU Biosafety Officer at (313) 577-1200.
11. Will the study involve use of No – go directly to Q# 12 chemotherapeutic (cytotoxic, anti- Yes neoplastic) drugs? a. Will work with these chemotherapeutic No drugs be performed in a WSU-owned Yes (SEE NOTE) laboratory? Provide Building and Room Number: NOTE: Use of certain biological agents requires review and approval by the WSU Institutional Biosafety Committee (IBC). To determine if this applies to your research, please contact the WSU Biosafety Officer at (313) 577-1200. The principal investigator is required to oversee the maintenance of the study drugs, including dates, quantity, and use by participants.
Appendix F Page 3 Form Date: 04/2015a Section B: Devices The FDA can assign a non-significant risk status for a device; however, the Institutional Review Board must assess the use of that device in a particular protocol. The IRB must do their own risk assessments for: 1. All devices used in a new protocol enrolling human participants in research and/or 2. When a non-approved device is used for single-time (compassionate) use.
See IRB Policy/Procedure: Approved and Non-Approved Devices 12. Will a medical device be used in this study? No – STOP, Appendix F is complete Yes a. Is the device being studied to evaluate No – STOP, Appendix F is complete its effectiveness and/or its safety? Yes 14. “ Non-significant risk ” device study: Does No – go directly to Q# 14 this device meet the criteria for a non- Yes significant risk device study?
For assistance, go to the FDA website: www.fda.gov/oc/ohrt/irbs/devices.html#risk a. Provide a justification from the sponsor Justification: why the device does not pose a significant risk, the IDE exemption category number, and/or a copy of all FDA or sponsor documents supporting this Exemption category #: justification. FDA/sponsor documents No are included with the Yes NOTE: If the IRB determines that the device is of submission: significant risk, an IDE number will be required. d. Briefly describe reports of prior investigations with this device. e. Abbreviated IDE Device review: Studies that Labeling: pose a Non-Significant Risk must comply with IRB Approval: the abbreviated review requirements Informed under 21 CFR 812.2(b). Please indicate your Consent: plans to comply with these requirements as Monitoring: follows: Records and NOTE: Further information and Reports: a description of these requirements is available in the “Handbook for Commercializa Investigators” available on the tion: IRB website. If more space is needed, please attach a separate page. 25. “ Significant risk” device study: Please Justification: provide justification for it’s use Does this device meet the criteria for a No – answer Q# 14a significant risk device study? Yes – answer Q# 14b a. If no, is this a new indication for the use No Appendix F Page 4 Form Date: 04/2015a of this device (e.g., new use, new Yes participant population)? b. If yes, is this an FDA approved device? No Yes 29. Provide the Investigational Device Number: Exemption (IDE) number and attach a copy Issue Date: of the IDE approval letter. No IDE number – answer Q# 16 NOTE: approval cannot be granted until receipt of the exemption number. 30. Exempt from requirement to have an IDE exemption category number: IDE: If the FDA has NOT provided an IDE Justification for exemption: number for the above indication, justify why their review is not required. Include the IDE exemption category number and/or a copy of all FDA or sponsor documents FDA or sponsor documents are attached supporting this justification. to support this justification? No NOTE: Refer to the IRB website, “Helpful Links”, Yes for FDA requirements: www.fda.gov/oc/ohrt/irbs/devices.html#risk If the IRB determines that the device is classified as significant risk, an IDE number will be required. 33. Describe the device accountability plan that includes receiving, storing, securing, dispensing, final disposition and accountability of the device.
NOTE: The principal investigator is required to oversee the maintenance of the device, including dates and use by participants, and disposal.
Appendix F Page 5 Form Date: 04/2015a