1
Supplementary information
Effect of acid and hydrothermal treatments on the dye adsorption properties of biomass-derived activated carbon
Guoting Zhua,b, Xianjun Xing b,c,*, Jiaquan Wang a, Xianwen Zhangb,c,* a School of Resources and Environment Engineering, Hefei University of Technology, Anhui, Hefei 230009, China b Institute of Advanced Energy Technology & Equipment, Hefei University of Technology, Anhui, Hefei 230009, China c School of Automobile and Transportation Engineering, Hefei University of Technology, Anhui, Hefei 230009, China
4 0 0 0 0 4 0 0 0 0
S a w d u s t w i t h P h o s p h o r i c A c i d 3 0 0 0 0 S a w d u s t 3 0 0 0 0 ) ) . . u u . . a a 2 0 0 0 0 2 0 0 0 0 ( ( y y
t t i i s s n n e e t t n n I 1 0 0 0 0 I 1 0 0 0 0
0 0 2 8 0 2 8 5 2 9 0 2 9 5 2 8 0 2 8 5 2 9 0 2 9 5 B E ( e v ) B E ( e v )
4 0 0 0 0 1 2 0 0 0 0
3 0 0 0 0 9 0 0 0 0 P h o s p h o r i c - A c i d - H T C P h o s p h o r i c - A c i d - H T C - 1 0 0 0 ) . ) . u u . .
a 6 0 0 0 0 a (
( 2 0 0 0 0 y y t
i t
i s s n n e e t t n n I I 1 0 0 0 0 3 0 0 0 0
0 0 2 8 0 2 8 5 2 9 0 2 9 5 2 8 0 2 8 5 2 9 0 2 9 5 B E ( e v ) B E ( e v )
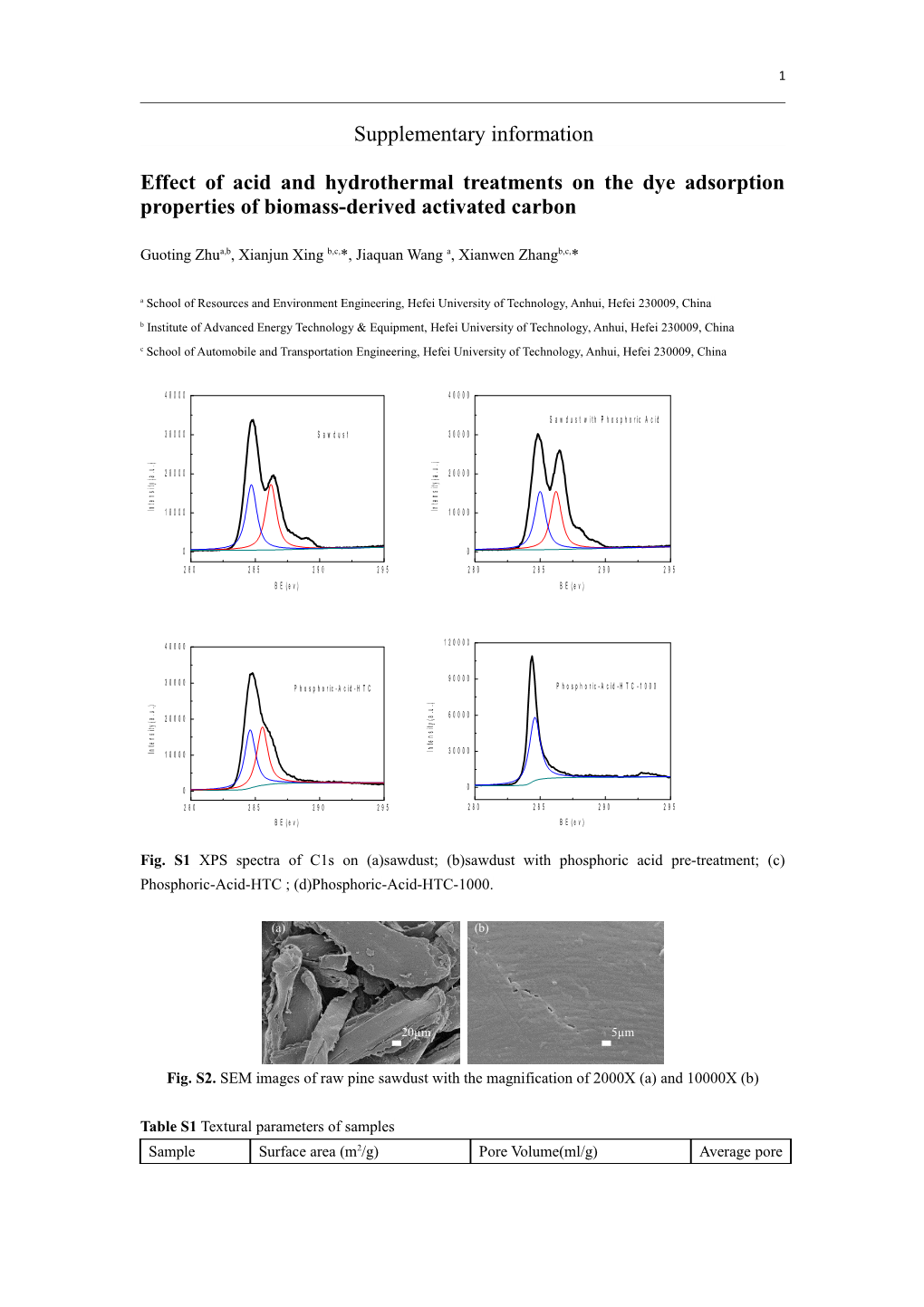
Fig. S1 XPS spectra of C1s on (a)sawdust; (b)sawdust with phosphoric acid pre-treatment; (c) Phosphoric-Acid-HTC ; (d)Phosphoric-Acid-HTC-1000.
(a) (b)
20µm 5µm
Fig. S2. SEM images of raw pine sawdust with the magnification of 2000X (a) and 10000X (b)
Table S1 Textural parameters of samples Sample Surface area (m2/g) Pore Volume(ml/g) Average pore 2
a b c d e Slangmuir Smicro Sexnal Vtotal Vmicro Vmeso width(nm) Phosphoric- 2254.24 1290.11 495.24 1.2699 0.7207 0.5492 3.3161 Acid-HTC- 1000 Tartaric-Acid- 1614.32 252.59 921.70 0.8007 0.4902 0.3105 3.0844 HTC-1000 Citric-Acid- 1319.16 156.16 778.58 0.7606 0.3686 0.392 3.2548 HTC-1000 Formic-Acid- 552.63 61.17 317.63 0.4736 0.02697 0.44663 5.0011 HTC-1000 a Measured using N2 adsorption with Langmuir method. b Micropore surface area calculated using the T-Plot method. c External surface area calculated using the T-Plot method. d Total pore volume determined at P/Po=0.99969561. e Micropore volume calculated using the DFT method.
Table S2 Methylene blue adsorption capacity of various ACs. Adsorbents Qm(mg/ References g) Bamboo dust-based 143.2 Dyes Pigments 51(2001)25-40 Jute fiber-based 225.6 J. Colloid Interface Sci. 284(2005)78-82 Coal-baesd 234 New Carbon Mater. 24(2009)141-146 Rice husk 40.58 J. Coll. Interface Sci.,286(2005)90-100 Pyrophyllite 70.42 J. Coll. Interface Sci.,286(2005)53-60 Pistacia khinjuk activated carbon 48-185 Ecotoxicol. Environ. Satety 96(2013) 110-117 Mesoporous materials synthesized 96.9 J. Ind. Eng. Chem. 20(2014)3667-3671 using waste quartz sand Maize stem tissue 160.84 J. Taiwan Inst. Chem. Eng. 45(2014) 1700- 1708 Polydopamine(PDA) microspheres 90.7 Chem. Eng. J. 259(2015)53-61 Sawdust activated carbon 303.03 This study commercial activated carbons(from 222.22 experiment Aladdin Industrial Corporation)
Table S3 Isotherm parameters for adsorption of MB on samples 3
Isotherm Parameter Adsorbent model Phosphoric- Tartaric-Acid- Citric-Acid- Formic-Acid- Acid-HTC- HTC-1000 HTC-1000 HTC-1000 1000
Langmuir qm ( mg/g 303.03 285.71 270.27 153.85 )
KL ( L/mg 3.30 3.18 4.63 1.97 ) R2 0.9923 0.9903 0.9915 0.9849
Freundlich KF(mg/g(L 185.95 191.58 219.86 91.15 /mg)1/n) n 3.2 0.19 3.34 3.62 R2 0.9710 0.9162 0.9769 0.9724
Reference
[1]N Kannan, MM Sundaram,2001. Kinetics and mechanism of removal of methylene blue by adsorption on various carbons— a comparative study.Dyes & Pigments, 51(1):25-40. [2]S Senthilkumaar, PR Varadarajan, K Porkodi, CV Subbhuraam, 2005. Adsorption of methylene blue onto jute fiber carbon: kin etics and equilibrium studies. J Colloid Interface Sci, 284(1):78-82. [3] GZ Gong,Q Xie,YF Zheng,YE Shu-Feng,YF Chen,2009. Regulation of pore size distribution in coal-based activated c arbon, 24(2):141-146. [4]V. Vadivelan and K. V. Kumar, 2005. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk.J. Coll. Interface Sci., 286 (1): 90-100. [5]A. Gucek, S. Sener, S. Bilgen and M.A. Mazmanci, 2005. Adsorption and kinetic studies of cationic and anionic dyes on pyro phyllite from aqueous solutions.J. Coll. Interface Sci., 286 (1): 53-60.
[6] M. Ghaedi, A.M. Ghaedi, F. Abdi, M. Roosta, A. Vafaei, A. Asghari, 2013. Principal component analysis-adaptive neuro- fuzzy inference system modeling and genetic algorithm optimization of adsorption of methylene blue by activated carbon derived from Pistacia khinjuk, Ecotoxicol. Environ. Safety 96 (2): 110–117. [7] J. M. Hong, B. Lin, J.S. Jiang, B.Y. Chen, C.T. Chang, 2014. Synthesis of poreexpanded mesoporous materials using waste quartz sand and the adsorption effects of methylene blue, J. Ind. Eng. Chem. 20 (5): 3667–3671. [8] V.M. Vucurovic , R.N. Razmovski, U.D. Miljic, V.S. Puskas, 2014. Removal of cationic and anionic azo dyes from aqueous solutions by adsorption on maize stem tissue, J. Taiwan Inst. Chem. Eng. 45 (4): 1700–1708 [9] J. Fu, Z. Chen, M. Wang, S. Liu, J. Zhang, J. Zhang, R. Han, Q. Xu, 2015. Adsorption of methylene blue by a high-efficiency adsorbent (polydopamine microspheres): kinetics, isotherm, thermodynamics and mechanism analysis, Chem. Eng. J. 259: 53–61.