AP/IB Chemistry Notes Name: Chapter 8 – Bonding: General Concepts
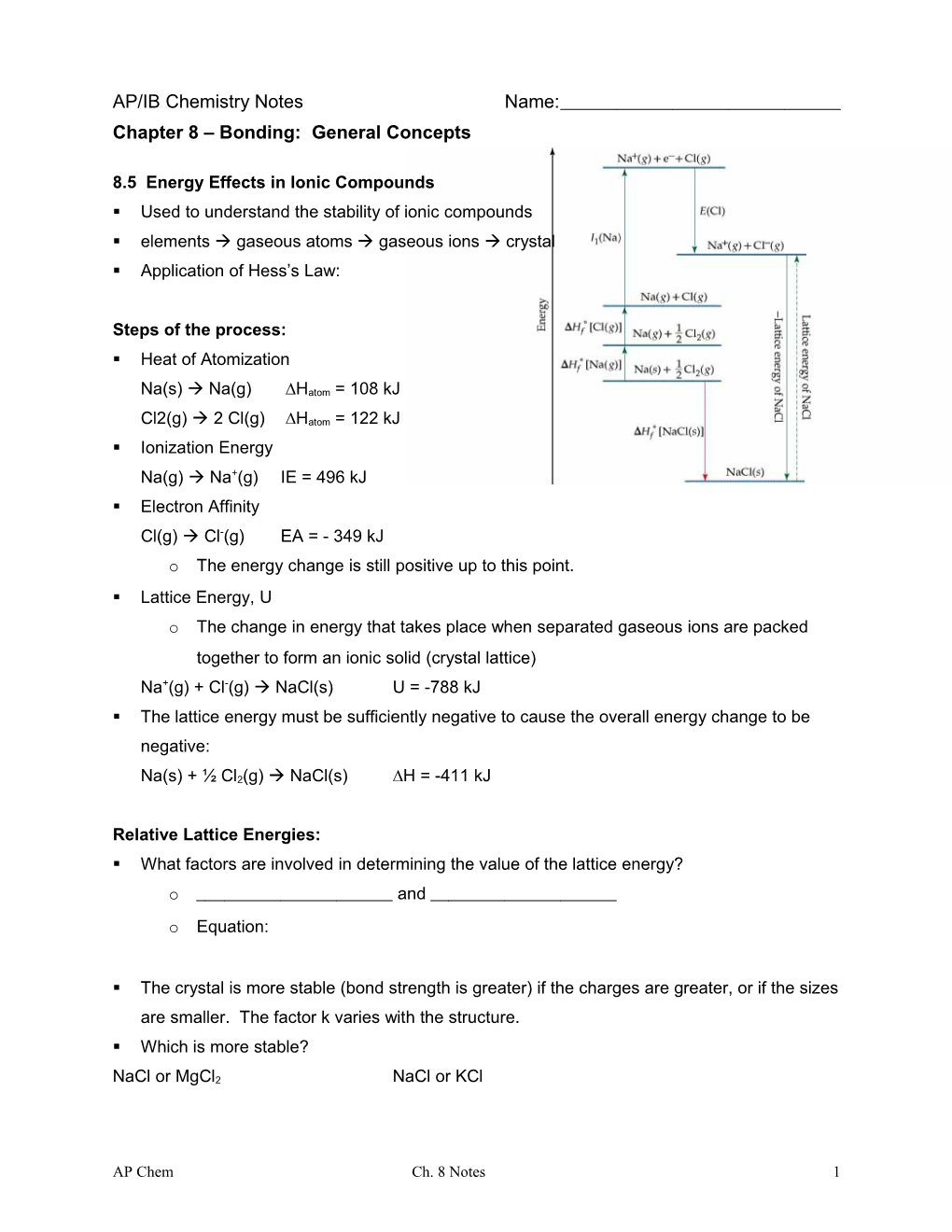
8.5 Energy Effects in Ionic Compounds . Used to understand the stability of ionic compounds . elements gaseous atoms gaseous ions crystal . Application of Hess’s Law:
Steps of the process: . Heat of Atomization
Na(s) Na(g) ∆Hatom = 108 kJ
Cl2(g) 2 Cl(g) ∆Hatom = 122 kJ . Ionization Energy Na(g) Na+(g) IE = 496 kJ . Electron Affinity Cl(g) Cl-(g) EA = - 349 kJ o The energy change is still positive up to this point. . Lattice Energy, U o The change in energy that takes place when separated gaseous ions are packed together to form an ionic solid (crystal lattice) Na+(g) + Cl-(g) NaCl(s) U = -788 kJ . The lattice energy must be sufficiently negative to cause the overall energy change to be negative:
Na(s) + ½ Cl2(g) NaCl(s) ∆H = -411 kJ
Relative Lattice Energies: . What factors are involved in determining the value of the lattice energy? o and o Equation:
. The crystal is more stable (bond strength is greater) if the charges are greater, or if the sizes are smaller. The factor k varies with the structure. . Which is more stable?
NaCl or MgCl2 NaCl or KCl
AP Chem Ch. 8 Notes 1 Na2O or MgO NaCl or NaBr 8.8 Covalent Bond Energy Considerations . Two pieces of evidence to consider: o Bond Energy (bond strength): o Bond Length (distance between atom centers): . Bond Energy or Bond Dissociation Energy - energy require to break a bond in a gaseous molecule o Reactions generally proceed to form compounds with more stable bonds (greater bond energy). Relate to Modern Lewis Model o Values in Table 8.4, pg 351
. Bond energy varies somewhat from one molecule to another, or even within one molecule, so we use an average bond energy
Bond Energy & Enthalpy of Reaction . Using bond energies to estimate energy changes in chemical processes: o Break all reactant bonds, then make product bonds
. Equation:
. Use only when heats of formation are not available, since bond energies are average values for gaseous molecules. . Why might this be a problem? . Use bond energies to calculate the enthalpy change for the following reaction:
N2(g) + 3H2(g) 2NH3(g)
. ∆Hrxn = ∆Hrxn = measured value = . Why are the values different?
AP Chem Ch. 8 Notes 2 . Sample Problem o Use bond energies to calculate the enthalpy change for the following reaction:
2CO(g) + O2(g) 2CO2(g)
Bond Energy & Bond Length . The distance between the nuclei of the atoms involved in a bond is called the bond length. o Multiple bonds are shorter than single bonds. o Multiple bonds are also stronger than single bonds. . As the number of bonds between two atoms increases, the atoms are held closer and more tightly together.
Summary: . Bond Energy (bond strength):
. provides support for Lewis Model . Bond Length (distance between atom centers):
AP Chem Ch. 8 Notes 3