Introduction & Figures of Merit
Suggested Reading Skoog/Holler/Crouch 6th Edition: Chapter 1A, 1B, 1E, Appendix 1 Harris 7th Edition: Chapters 3&4, mostly same material as Appendix 1 of Skoog, Chapter 5 Sections 3&4, Appendix B&C Some of this material is redundant between the two texts
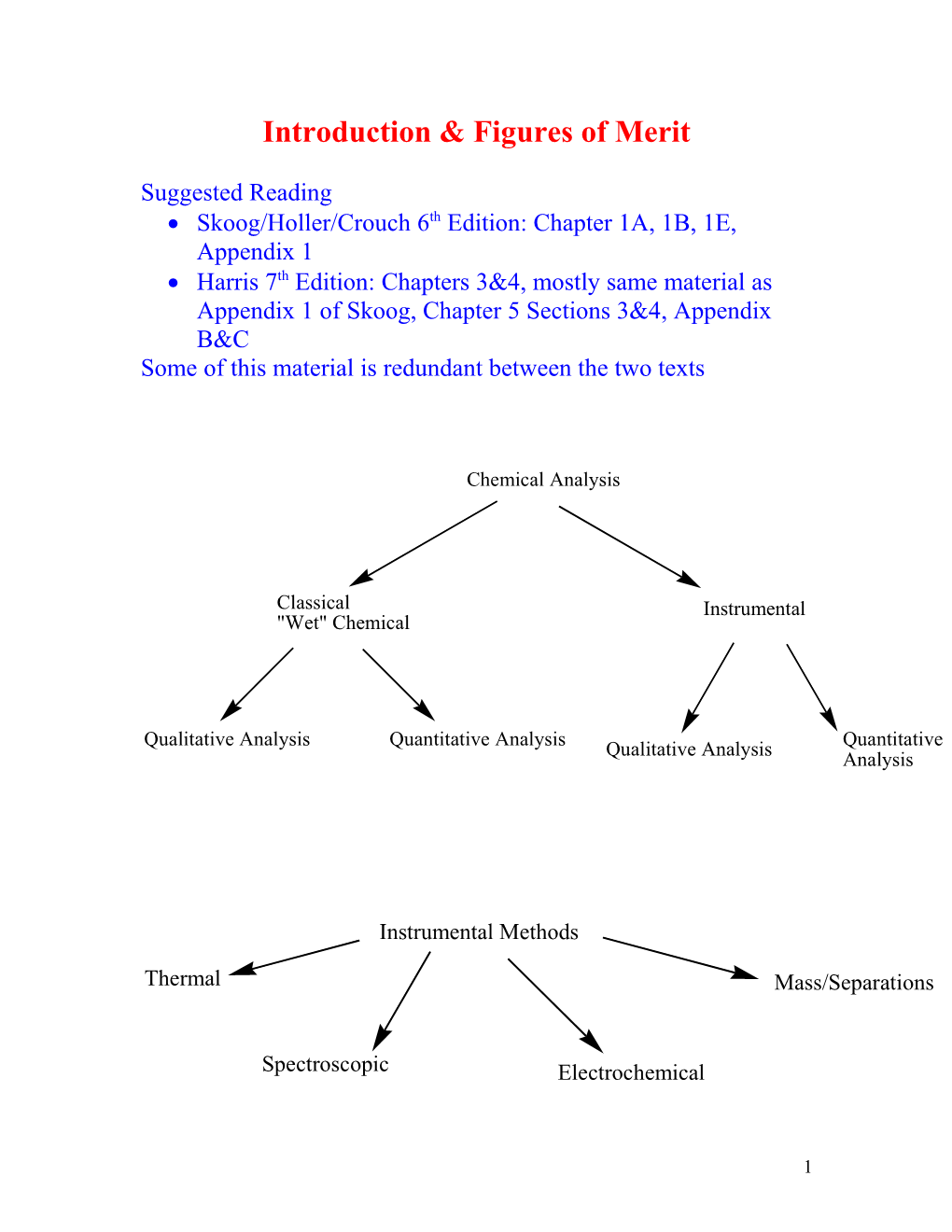
Chemical Analysis
Classical Instrumental "Wet" Chemical
Qualitative Analysis Quantitative Analysis Qualitative Analysis Quantitative Analysis
Instrumental Methods
Thermal Mass/Separations
Spectroscopic Electrochemical
1 To perform an analysis, the analyte must often first be separated from a mixture.
Analytical Separations
Chromatography Mass Spectrometry GC, LC, SFC Capillary Electrophoresis
After separation chemical analysis often performed on analyte.
Preparatory Separations – analysis often performed off-line. (Synthesis, large scale separations) Analytical Separations – analysis often performed on-line with separation method. This course is concerned with analytical, or very small scale separations.
Fundamentals are the same for analytical and preparatory separations, technique specifics differ.
General Course Outline
1. Fundamentals A. Instrument performance characteristics (Figures of Merit) and statistical background review.
B. Instrument Calibrations
2 a. Traditional (Review) b. Standard Additions c. Internal Standards C. Signals, Noise, S/N a. Definitions/Sources b. Methods for S/N enhancement
2. Separations A. Mass Spectrometry a. Fundamentals b. Instrumentation c. Information
B. Chromatography a. Fundamentals – Plate and Rate Theories, etc. b. Liquid and Gas chromatography c. Capillary electrophoresis
3. Analytical Spectroscopy A. Fundamentals of absorption/emission processes a. Line, Band, Continuous spectra b. Measurement basics of absorption and emission spectra & types of instrumentation
B. Absorption spectroscopy a. Molecular (UV-vis, IR) b. Atomic (AA)
C. Emission spectroscopy a. Atomic (AES) b. Molecular (Fluorescence)
General Instrument Performance Characteristics
3 First define the problem: What accuracy is required? What is the analyte concentration range? What components of the sample might interfere with the analysis? Etc.
Tables 1-3, 1-5 (Skoog)
1. Precision = Measurement Reproducibility Review statistics:
2. Bias = systematic measurement error
4 3. Sensitivity = Ability to distinguish between small differences in analyte concentration
Sensitivity Illustration
12
S = mC + SBl
10
8 l a n g i S
t
n 6 e m u r t s n I
4
2
0 0 1 2 3 4 5 6 Analyte Concentration
4. Detection Limit = minimum analyte concentration that can be detected with a given confidence level.
5 SUMMARY OUTPUT Standard Coefficients Error Regression Statistics Intercept 1.517857143 0.294936001 X Variable Multiple R 0.998879565 1 1.930357143 0.040900264 R Square 0.997760386 Adjusted R Square 0.997312463 Standard Error 0.432847713 Observations 7
Find the detection limit for a fluorescence analysis given the following calibration data. Analyte Conc. (pg/mL) Fluorescence Intensity 0 2.1 2 5.0 4 9.0 6 12.6 8 17.3 10 21 12 24.7
30
l y = 1.9304x + 1.5179 a 25 n g i
S 20
e c
n 15 e c s
e 10 r o u
l 5 F 0 0 2 4 6 8 10 12 14 pg/mL
6 5. Linear Dynamic Range = Analyte concentration range from the Limit of Quantification concentration at which there is a non- linear instrument response.
7 6. Selectivity = A method’s ability to selectively analyze an analyte in the presence of other chemical species (the sample “matrix”). Signal = maCa + mbCb + Sbl m = analytical sensitivity, c = concentration
8