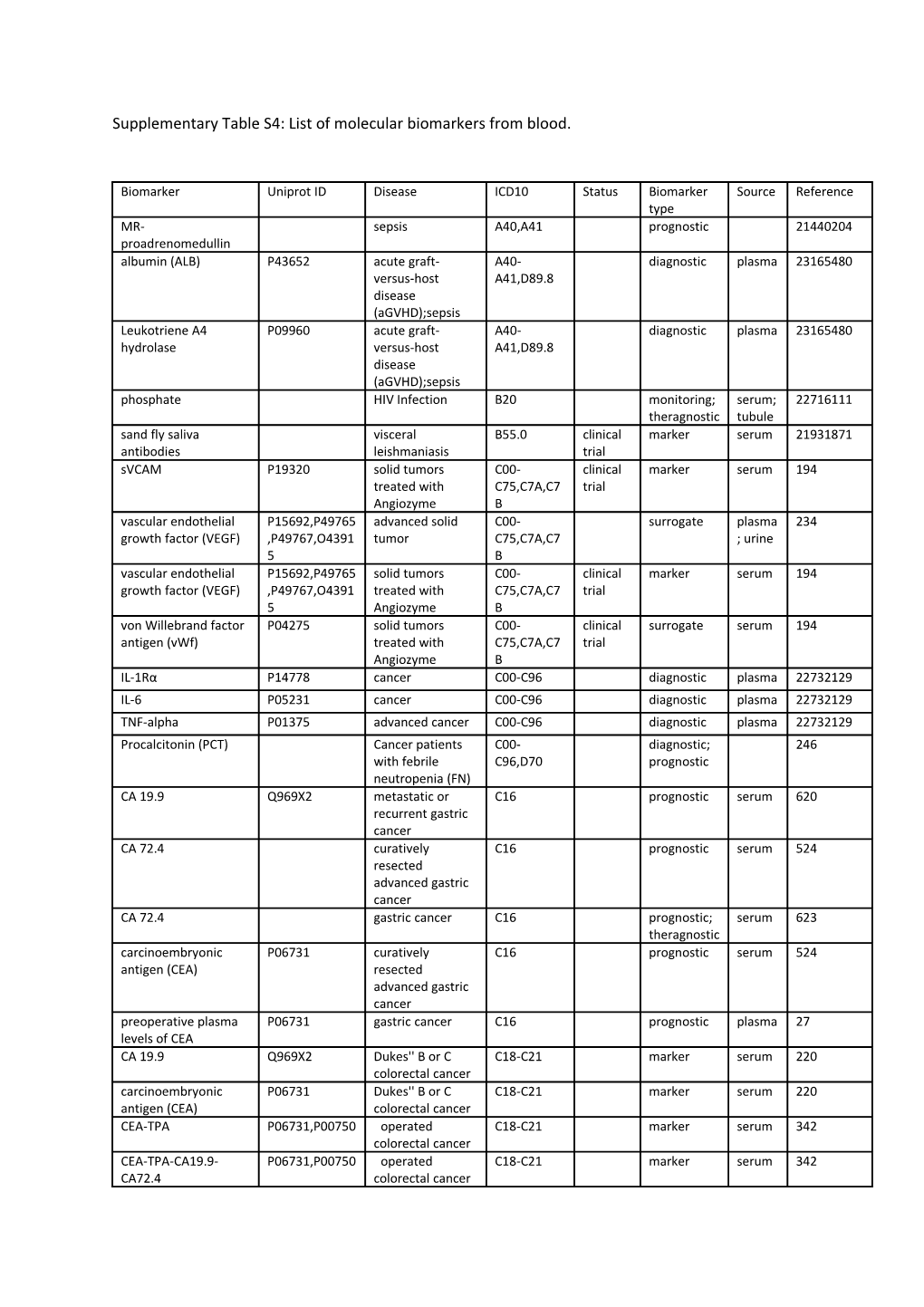
Supplementary Table S4: List of molecular biomarkers from blood.
Biomarker Uniprot ID Disease ICD10 Status Biomarker Source Reference type MR- sepsis A40,A41 prognostic 21440204 proadrenomedullin albumin (ALB) P43652 acute graft- A40- diagnostic plasma 23165480 versus-host A41,D89.8 disease (aGVHD);sepsis Leukotriene A4 P09960 acute graft- A40- diagnostic plasma 23165480 hydrolase versus-host A41,D89.8 disease (aGVHD);sepsis phosphate HIV Infection B20 monitoring; serum; 22716111 theragnostic tubule sand fly saliva visceral B55.0 clinical marker serum 21931871 antibodies leishmaniasis trial sVCAM P19320 solid tumors C00- clinical marker serum 194 treated with C75,C7A,C7 trial Angiozyme B vascular endothelial P15692,P49765 advanced solid C00- surrogate plasma 234 growth factor (VEGF) ,P49767,O4391 tumor C75,C7A,C7 ; urine 5 B vascular endothelial P15692,P49765 solid tumors C00- clinical marker serum 194 growth factor (VEGF) ,P49767,O4391 treated with C75,C7A,C7 trial 5 Angiozyme B von Willebrand factor P04275 solid tumors C00- clinical surrogate serum 194 antigen (vWf) treated with C75,C7A,C7 trial Angiozyme B IL-1Rα P14778 cancer C00-C96 diagnostic plasma 22732129 IL-6 P05231 cancer C00-C96 diagnostic plasma 22732129 TNF-alpha P01375 advanced cancer C00-C96 diagnostic plasma 22732129 Procalcitonin (PCT) Cancer patients C00- diagnostic; 246 with febrile C96,D70 prognostic neutropenia (FN) CA 19.9 Q969X2 metastatic or C16 prognostic serum 620 recurrent gastric cancer CA 72.4 curatively C16 prognostic serum 524 resected advanced gastric cancer CA 72.4 gastric cancer C16 prognostic; serum 623 theragnostic carcinoembryonic P06731 curatively C16 prognostic serum 524 antigen (CEA) resected advanced gastric cancer preoperative plasma P06731 gastric cancer C16 prognostic plasma 27 levels of CEA CA 19.9 Q969X2 Dukes'' B or C C18-C21 marker serum 220 colorectal cancer carcinoembryonic P06731 Dukes'' B or C C18-C21 marker serum 220 antigen (CEA) colorectal cancer CEA-TPA P06731,P00750 operated C18-C21 marker serum 342 colorectal cancer CEA-TPA-CA19.9- P06731,P00750 operated C18-C21 marker serum 342 CA72.4 colorectal cancer Cytokeratin 18 (CK18) P05783 metastatic C18-C21 prognostic; plasma 549 colorectal cancer theragnostic (mCRC) DcR3 (DD-C248) O95407 colon cancer C18-C21 diagnostic serum 419 glycodelin P09466 metastatic C18-C21 clinical marker serum 386 colorectal cancer trial IGF-binding protein-3 P17936 metastatic C18-C21 clinical prognostic; plasma 19073970 (IGFBP-3) colorectal cancer trial theragnostic MASP-2 O00187 Dukes''B or C18-C21 classificatio serum; 357 Dukes''C colon n; plasma cancer theragnostic metalloproteinases 1 P01033 advanced C18-C21 clinical monitoring plasma 417 (TIMP-1) colorectal cancer trial p53 antigen P04637 colon cancer C18-C21 detective serum 49 pro-enzyme form of P03956 Colon Cancer C18-C21 marker serum 108 human collagenase-1 (ProMMP-1) Reg IV (DD-C101) Q9BYZ8 colon cancer C18-C21 diagnostic serum 419 Spondin-2 (DD-P108) Q9BUD6 colon cancer C18-C21 diagnostic serum 419 TRAIL-R2 O14763 colon cancer C18-C21 diagnostic serum 419 tumor type M2 P14618 advanced C18-C21 clinical monitoring plasma 417 pyruvate kinase colorectal cancer trial (TuM2-PK) vascular endothelial P15692,P49765 Colorectal C18-C21 prognostic serum 85 growth factor (VEGF) ,P49767,O4391 Carcinoma 5 tissue inhibitor of Dukes''B or C18-C21 classificatio serum; 357 metalloproteinase Dukes''C colon n; plasma (TIMP 1) cancer theragnostic Carboxyethylpyrrole metastatic C18-C21 prognostic 600 (CEP) colorectal cancer (mCRC) sialyl Lewis a (sLea) colorectal cancer C18-C21 prognostic 592 (CCR) sialyl Lewis x (sLex) colorectal cancer C18-C21 prognostic 592 (CCR) D-dimer metastatic C18-C21 prognostic 568 colorectal cancer urokinase-type P00749 BREAST CANCER, C18- marker plasma 67 plasminogen activator COLON CANCER C21,C50 (uPA) PlGF P49763 rectal cancer (RC) C20 clinical prognostic plasma 480 trial vascular endothelial P15692,P49765 rectal cancer (RC) C20 clinical prognostic plasma 480 growth factor (VEGF) ,P49767,O4391 trial 5 alpha-fetoprotein P02771 hepatocellular C22.0 phase III prognostic; serum 19064965 (AFP) carcinoma monitoring; theragnostic glypican 3 (GPC3) hepatocellular C22.0 detective 450 cancer (HCC) CA 19.9 Q969X2 advanced C25 clinical surrogate; serum 171 pancreatic cancer trial monitoring; theragnostic Cytokeratin 18 (CK18) P05783 pancreatic cancer C25 prognostic plasma 488 regenerating islet- Q9BYZ8 Pancreatic cancer C25 diagnostic serum 466 derived 4 (REG4) (PC) ACE P12821 stage I-III NSCLC C33,C34 clinical prognostic serum 378 trial bFGF P09038 stage I non-small C33,C34 prognostic serum 305 cell lung cancer CA 125 Q8WXI7 non-small cell C33,C34 prognostic; serum 51 lung cancer theragnostic carcinoembryonic P06731 non-small cell C33,C34 prognostic; serum 51 antigen (CEA) lung cancer theragnostic CYFRA non-small cell C33,C34 prognostic; serum 51 lung cancer theragnostic IL-10 P22301 non-small-cell C33,C34 diagnostic plasma 22732129 lung cancer with concurrent chemoradiation IL-6 P05231 non-small-cell C33,C34 diagnostic plasma 22732129 lung cancer with concurrent chemoradiation NSE Q96KN4 non-small cell C33,C34 prognostic; serum 51 lung cancer theragnostic Osteopontin (OPN) P10451 advanced non- C33,C34 clinical prognostic plasma 244 small cell lung trial cancer (NSCLC) plasminogen activator P05121 advanced non- C33,C34 clinical prognostic plasma 244 inhibitor 1 (PAI-1) small cell lung trial cancer (NSCLC) sTNF-R1 P19438,P20333 non-small-cell C33,C34 diagnostic plasma 22732129 lung cancer with concurrent chemoradiation UPAR Q03405 stage I non-small C33,C34 prognostic serum 305 cell lung cancer vascular endothelial P15692,P49765 advanced non- C33,C34 clinical prognostic plasma 244 growth factor (VEGF) ,P49767,O4391 small cell lung trial 5 cancer (NSCLC) vascular endothelial P15692,P49765 stage I non-small C33,C34 prognostic serum 305 growth factor (VEGF) ,P49767,O4391 cell lung cancer 5 cross-linked Primary Lung C33,C34 marker serum 154 carboxyterminal Cancer telopeptide of Type I collagen (ICTP) antigen A RESECTED STAGE I C33,C34 prognostic 64 AND II NON- SMALL CELL LUNG CANCER neuron specific P09104 small cell lung C34.90 phase III prognostic serum 11 enolase cancer Pro-gastrin-releasing small cell lung C34.90 diagnostic serum 12 peptide (31-98) cancer Melanoma-Inhibiting Q16674 Metastatic C43 clinical marker serum 102 Activity (MIA) Melanoma trial S100B P04271 Metastatic C43 clinical marker serum 102 Melanoma trial sIL-2R P01589,P14784 melanoma C43 clinical prognostic serum 20454974 ,P31785 trial TNF-alpha P01375 melanoma C43 clinical prognostic serum 20454974 trial YKL-40 P36222 metastatic C43 prognostic serum 327 malignant melanoma β-2 microglobulin P61769 melanoma C43 clinical prognostic serum 20454974 (B2M) trial NKG2D ligand metastatic C43 phase II associative 602 melanoma NY-ESO-1 response to anti- C43 prognostic; 514 CTLA-4 theragnostic (Ipimilumab) treatment Osteopontin (OPN) P10451 head and neck C44 prognostic serum 174 squamous cell carcinomas (HNSCC) CA 125 Q8WXI7 Malignant Pleural C45 prognostic serum 114 Mesothelioma carcinoembryonic P06731 Malignant Pleural C45 marker serum 114 antigen (CEA) Mesothelioma CYFRA 21-1 Malignant Pleural C45 prognostic serum 114 Mesothelioma epidermal growth P01133 malignant pleural C45 prognostic serum 445 factor (EGF) mesothelioma megakaryocyte Q13421 malignant pleural C45 monitoring serum 649 potentiating factor mesothelioma (MPF) Osteopontin (OPN) P10451 malignant pleural C45 prognostic plasma 649 mesothelioma platelet-derived P16234,P09619 malignant pleural C45 prognostic serum 445 growth factor (PDGF- mesothelioma AB) Soluble mesothelin Q13421 malignant pleural C45 monitoring serum 649 (SM) mesothelioma BONE SIALOPROTEIN P21815 PRIMARY BREAST C50 marker serum 70 (BSP) CANCER CA 15.3 P15941 Metastatic Breast C50 clinical prognostic; serum 159 Cancer trial monitoring carcinoembryonic P06731 metastatic breast C50 prognostic serum 215 antigen (CEA) cancer patients treated with 1st- line hormone therapy carcinoembryonic P06731 breast cancer C50 prognostic serum 394 antigen (CEA) CEA-TPA-CA15.3 P06731,P00750 breast cancer C50 marker serum 268 ,P15941 Colony-stimulating P09603 breast cancer C50 marker serum 560 factor-1 (CSF-1) dynein breast cancer C50 surrogate; periph 537 theragnostic eral blood folliculin Q8NFG4 breast cancer C50 surrogate; periph 537 theragnostic eral blood HER2/neu P04626 Metastatic Breast C50 clinical prognostic serum 159 Cancer trial HER2/neu P04626 metastatic breast C50 prognostic serum 215 cancer patients treated with 1st- line hormone therapy IL-6 P05231 breast cancer C50 marker serum 90 IL-8 P10145 metastatic breast C50 prognostic plasma 572 cancer Krebs Von Den Lungen P15941 Breast Cancer C50 marker serum 143 6 (KL-6) Osteopontin (OPN) P10451 metastatic breast C50 prognostic plasma 243 cancer Tartrate-resistant acid P13686 breast carcinoma C50 clinical surrogate serum 272 phosphatase 5b (TRAP trial 5b) TNF receptor Q12931 breast cancer C50 surrogate; periph 537 associated protein 1 theragnostic eral blood trefoil factor 1 P04155 breast cancer C50 surrogate; periph 537 theragnostic eral blood glucocorticoids breast cancer C50 diagnostic 22732129 survivors N-terminal pro-B-type HER-2-positive C50 prognostic 289 natriuretic peptide metastatic breast (NT-pro-BNP) cancer the amino-terminal Advanced breast C50 phase III marker 343 procollagen cancer propeptides of type I collagen (P1NP) C-terminal telopeptide Advanced breast C50 phase III marker 343 of type I collagen in cancer serum (S-CTX) vascular endothelial P15692,P49765 squamous cell C53 prognostic serum 340 growth factor (VEGF) ,P49767,O4391 carcinoma of the 5 uterine cervix VEGF-C P49767 squamous cell C53 prognostic serum 340 carcinoma of the uterine cervix YKL-40 P36222 adenocarcinoma C53 diagnostic serum 456 of the uterine cervix EGFR P00533 persistent or C54.1 clinical prognostic serum 554 recurrent trial endometrial cancer BETA 2- P61769 advanced C56 prognostic; serum 53 MICROGLOBULIN epithelial ovarian theragnostic cancer CA 125 Q8WXI7 ovarian cancer C56 clinical prognostic serum 150 trial Cln101 ovarian cancer C56 detective serum 254 E-selectin P16581 ovarian cancer C56 clinical surrogate serum 278 trial Ovr110 ovarian cancer C56 detective serum 254 p55 Q8N3R9 ovarian cancer C56 prognostic serum 50 p75 P14317 ovarian cancer C56 prognostic serum 50 vascular cell adhesion P19320 ovarian cancer C56 clinical surrogate serum 278 molecule-1 (VCAM-1) trial YKL-40 P36222 Recurrent Ovarian C56 prognostic plasma 136 Cancer CA 19-9 epithelial ovarian C56 prognostic serum 30 cancer stage I and II Serum Tumor Markers PN1, N2 and N3 C56, C62, prognostic serum 74 (STM) pre-RPLND Nonseminomatou D27, D29.2 s Germ Cell Tumor (NSGCT) human chorionic P01215,P01233 placental tumor C58 marker serum 94 gonadotrophin (hCG) Bone specific alkaline P05186 advanced C61 marker serum 216 phosphatase (bALP) prostate cancer fPSA/tPSA P07288 prostate cancer C61 diagnostic serum 494 IGF-binding protein-3 P17936 Metastatic C61 marker serum 144 (IGFBP-3) Prostate Cancer (PCA) IL-6 P05231 ANDROGEN- C61 prognostic serum 56 INDEPENDENT PROSTATE CANCER (AIPC) matrix P14780 metastatic C61 clinical marker serum 285 metalloproteinase 9 hormone trial (MMP 9) resistant prostatic cancer (HRPC) osteocalcin (OC) P02818 prostate cancer C61 clinical prognostic serum 636 trial OVX1 androgen- C61 prognostic serum 6 independent prostate cancer P1NP prostate cancer C61 associative serum 528 PAP P51003 metastatic C61 clinical marker serum 285 hormone trial resistant prostatic cancer (HRPC) prostate-specific P07288 prostate cancer C61 clinical antecedent serum 18006214 antigen (PSA) trial prostate-specific P07288 metastatic C61 clinical marker serum 285 antigen (PSA) hormone trial resistant prostatic cancer (HRPC) TRAP P13686 prostate cancer C61 associative serum 528 amino-terminal prostate cancer C61 diagnostic serum 425 procollagen propeptides of type 1 collagen (PINP) Pyridinoline cross- prostate cancer C61 associative serum 528 linked carboxyterminal telopeptide of type I collagen (1CTP) procollagen amino- prostate cancer C61 marker serum 564 terminal propeptide 1 (PC) (P1NP) chromogranin A (CGA) metastatic C61 clinical marker serum 285 hormone trial resistant prostatic cancer (HRPC) deoxy-pyridinoline hormone C61 Phase II prognostic 316 (DPD) refractory prostate cancer (HRPC) pyridinoline [PYD] hormone C61 Phase II prognostic 316 refractory prostate cancer (HRPC) N-telopeptide (NTX) hormone C61 Phase II prognostic 316 refractory prostate cancer (HRPC) procollagen amino- hormone C61 Phase II prognostic 316 terminal propeptide 1 refractory (P1NP) prostate cancer (HRPC) D-dimer chemotherapy C61 marker 446 refractory prostate cancer (CRefracPC) N-telopeptide (NTX) advanced C61 marker 216 prostate cancer N-terminal propeptide breast cancer; C61,C50 diagnostic serum 486 of type I collagen prostate cancer (NTX) N-terminal propeptide breast cancer; C61,C50 diagnostic serum 531 of type I collagen prostate cancer (NTX) peptide-bound breast cancer; C61,C50 diagnostic serum 531 collagen type I cross- prostate cancer links C-telopeptides (ICTP) peptide-bound breast cancer; C61,C50 diagnostic serum 486 collagen type I cross- prostate cancer links C-telopeptides (ICTP) deoxy-pyridinoline bone metastatic C61,C79.51 marker 100 (DPD) prostate cancer deoxy-pyridinoline bone metastatic C61,C79.51 prognostic 100 (DPD) prostate cancer C-reactive protein P02741 testcular cancer, C62 diagnostic plasma 22732129 (CRP) long term survivors IL-1Rα P14778 testcular cancer, C62 diagnostic plasma 22732129 long term survivors alpha-fetoprotein P02771 non- C62,D29.2 clinical prognostic serum 185 (AFP) seminomatous trial germ-cell tumors (NSGCT) alpha-fetoprotein P02771 Primary C62,D29.2 prognostic serum 501 (AFP) mediastinal non- seminomatous germ cell tumors (PMNSGT) alpha-fetoprotein P02771 primary C62,D29.2 prognostic serum 479 (AFP) mediastinal nonseminomatou s germ cell tumor (PMNSGCT) human chorionic P01215,P01233 non- C62,D29.2 clinical prognostic serum 185 gonadotrophin (hCG) seminomatous trial germ-cell tumors (NSGCT) human chorionic P01215,P01233 Primary C62,D29.2 prognostic serum 501 gonadotrophin (hCG) mediastinal non- seminomatous germ cell tumors (PMNSGT) human chorionic P01215,P01233 primary C62,D29.2 prognostic serum 479 gonadotrophin (hCG) mediastinal nonseminomatou s germ cell tumor (PMNSGCT) human chorionic P01215,P01233 clinical Stage A C62,D29.2 classificatio serum 20 gonadotrophin (hCG) testicular n nonseminomatou s germ cell tumors (NSGCT) a-Fetoprotein (AFP) P02771 advanced C62.10; Clinical monitoring serum 21037873 seminoma C62.90 Society Recom mended human chorionic P01215,P01233 advanced C62.10; Clinical monitoring serum 21037873 gonadotrophin (hCG) seminoma C62.90 Society Recom mended matrix P14780 metastatic renal C64 prognostic serum 530 metalloproteinase 9 cell carcinoma (MMP 9) (MRCC) TNF-alpha P01375 metastatic renal C64 prognostic serum 530 cell carcinoma (MRCC) beta HCG P01233 advanced bladder C67 prognostic; serum 63 cancer theragnostic CA 125 Q8WXI7 advanced bladder C67 prognostic; serum 63 cancer theragnostic CA 19.9 Q969X2 advanced bladder C67 prognostic; serum 63 cancer theragnostic carcinoembryonic P06731 advanced bladder C67 prognostic; serum 63 antigen (CEA) cancer theragnostic Serum tissue bladder C67 prognostic serum 47 polypeptide antigen carcinoma (S-TPA) C-reactive protein P02741 breast & prostate C67,C50 diagnostic plasma 22732129 (CRP) cancer with radiation IL-1Rα P14778 breast & prostate C67,C50 diagnostic plasma 22732129 cancer with radiation pamidronate breast and C67,C50 marker 34 prostate cancer IGFBP-2 P18065 Glioblastoma C71 diagnostic serum 402 multiforme (GBM) matrix P14780 GBM;anaplastic C71 monitoring serum 506 metalloproteinase 9 astrocytoma (AA) (MMP 9) urokinase-type P00749 recurrent C71 prognostic serum 562 plasminogen activator malignant glioma (uPA) YKL-40 P36222 high-grade C71 prognostic serum 506 gliomas (HGG) ferritin P02792,P02794 Patients >=18 C74 prognostic serum 472 ,Q8N4E7 months of age at diagnosis of INSS Stage 3 MYCN-NA NB 5-hydroxytryptamine carcinoid tumor C75, E34.0 marker 59 (5-HT) Serum bone-specific P05186 Extent of C79.51 marker serum 79 alkaline phosphatase Metastatic Bone (B-AP) Disease peptide-bound Extent of C79.51 marker serum 79 collagen type I cross- Metastatic Bone links C-telopeptides Disease (ICTP) carboxyterminal bone metastases C79.51 prognostic serum 370 telopeptide (ICTP) (BM) N-telopeptide (NTX) bone metastases C79.51 prognostic serum; 16 plasma ; urine deoxy-pyridinoline bone metastase C79.51 clinical prognostic 211 (DPD) trial pyridinoline (PYD) bone metastase C79.51 clinical prognostic 211 trial β-crosslaps bone metastases C79.51 phase II prognostic 458 N-telopeptide (NTX) bone metastase C79.51 clinical prognostic 211 trial N-telopeptide of type I patients (pts) with C79.51 clinical marker 437 collagen (NTX) bone metastase trial Procollagen-I- bone metastases C79.51 phase II prognostic 458 propeptide N-terminal propeptide lung cancer with C79.51,C33- monitoring; 601 of type I collagen bone metastasis C34 theragnostic (NTX) Bone specific alkaline P05186 breast cancer C79.51,C50 associative serum 622 phosphatase (bALP) with bone metastases MMP1 P03956 breast cancer C79.51,C50 prognostic serum 471 patients (BC) with bone metastases (BM) peptide-bound breast cancer C79.51,C50 prognostic serum 471 collagen type I cross- patients (BC) with links C-telopeptides bone metastases (ICTP) (BM) cross-linked N- breast cancer C79.51,C50 monitoring; serum 582 telopeptide of type I with bone theragnostic collagen (NTX) metastases cortisol ambulatory C79.51,C50 clinical marker serum; 304 breast cancer trial urine patients with bone metastase deoxy-pyridinoline ambulatory C79.51,C50 clinical marker serum; 304 (DPD) breast cancer trial urine patients with bone metastase N-telopeptide (NTX) ambulatory C79.51,C50 clinical marker serum; 304 breast cancer trial urine patients with bone metastase a cross-linked, beta- ambulatory C79.51,C50 clinical marker serum; 304 aspartate-isomerized breast cancer trial urine form of the epitope patients with EKAHDGGR derived bone metastase from the carboxyterminal telopeptide region of type I collagen alpha(1) chain vascular endothelial P15692,P49765 Hodgkin''s diseas C81 prognostic serum 399 growth factor (VEGF) ,P49767,O4391 5 Soluble interleukin-2 P01589,P14784 malignant C81-C96 prognostic serum 18 receptor lymphomas Immunoglobulin free P01708 Waldenstrom''s C88.0 clinical prognostic; serum 478 light chain macroglobulinemi trial theragnostic a CA 15.3 P15941 multiple myeloma C90.0 clinical prognostic serum 166 (MM) trial DPD Q12882 myeloma bone C90.0 diagnostic serum; 330 disease urine Hepatocyte Growth P14210 multiple myeloma C90.0 associative periph 281 Factor (HGF) eral blood; bone marro w Osteopontin (OPN) P10451 multiple myeloma C90.0 prognostic serum 499 a cross-linked, beta- myeloma bone C90.0 diagnostic serum; 330 aspartate-isomerized disease urine form of the epitope EKAHDGGR derived from the carboxyterminal telopeptide region of type I collagen alpha(1) chain Cardiac Troponin T P45379 Childhood Acute C91.0 marker serum 73 Lymphoblastic Leukemia N-terminal pro-B-type children with C91.0 diagnostic 598 natriuretic peptide Acute (NT-pro-BNP) Lymphoblastic Leukemia (ALL) serum iron Iron deficiency D50 marker serum 23687454 caused anemia albumin (ALB) P43652 graft-versus-host D89.8 prognostic plasma 23165480 disease (GVHD) albumin (ALB) P43652 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum albumin (ALB) P43652 acute graft- D89.8 monitoring plasma 22927351 versus-host ; disease (aGVHD) serum albumin (ALB) P43652 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum CCL5 P13501 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum CCL8 P80075 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum CCL8 P80075 acute graft- D89.8 antecedent plasma 22927351 versus-host ; disease (aGVHD) serum CCL8 P80075 acute graft- D89.8 prognostic plasma 22927351 versus-host ; disease (aGVHD) serum CK18Fs P05783 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum CK18Fs P05783 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum Collagen α-1 P20908 acute graft- D89.8 diagnostic plasma 23165480 versus-host disease (aGVHD) C-reactive protein P02741 graft-versus-host D89.8 prognostic plasma 23165480 (CRP) disease (GVHD) CXCL10 P02778 acute graft- D89.8 diagnostic plasma 23165480 versus-host disease (aGVHD) CXCL10 P02778 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum CXCL10 TNFR1 P02778,P19438 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum Elafin P19957 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum Elafin P19957 acute graft- D89.8 monitoring plasma 22927351 versus-host ; disease (aGVHD) serum Elafin P19957 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum GRO-α P09341 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum GRO-α P09341 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum HGF P14210 acute graft- D89.8 prognostic plasma 23165480 versus-host disease (aGVHD) HGF P14210 acute graft- D89.8 diagnostic plasma 23165480 versus-host disease (aGVHD) HGF P14210 acute graft- D89.8 diagnostic plasma 23165480 versus-host disease (aGVHD) HGF P14210 graft-versus-host D89.8 prognostic plasma 23165480 disease (GVHD) HGF P14210 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum HGF P14210 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum HGF P14210 acute graft- D89.8 monitoring plasma 22927351 versus-host ; disease (aGVHD) serum IFNγ P01579 acute graft- D89.8 antecedent plasma 22927351 versus-host ; disease (aGVHD) serum IFNγ P01579 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum IL-10 P22301 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum IL-10 P22301 acute graft- D89.8 monitoring plasma 22927351 versus-host ; disease (aGVHD) serum IL-10 P22301 aGVHD, chronic D89.8 diagnostic plasma 23165480 GVHD (cGVHD), infection, relapse IL-12 Q99665 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum IL-12 Q99665 acute graft- D89.8 diagnostic plasma 23165480 versus-host disease (aGVHD) IL-12 Q99665 acute graft- D89.8 antecedent plasma 22927351 versus-host ; disease (aGVHD) serum IL-12 Q99665 acute graft- D89.8 prognostic plasma 22927351 versus-host ; disease (aGVHD) serum IL-15 P40933 acute graft- D89.8 antecedent plasma 22927351 versus-host ; disease (aGVHD) serum IL-15 P40933 acute graft- D89.8 monitoring plasma 22927351 versus-host ; disease (aGVHD) serum IL-15 P40933 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum IL-18 Q14116 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum IL-18 Q14116 acute graft- D89.8 monitoring plasma 22927351 versus-host ; disease (aGVHD) serum IL-18 Q14116 acute graft- D89.8 antecedent plasma 22927351 versus-host ; disease (aGVHD) serum IL-18 Q14116 acute graft- D89.8 prognostic plasma 22927351 versus-host ; disease (aGVHD) serum IL-18 Q14116 acute graft- D89.8 diagnostic plasma 23165480 versus-host disease (aGVHD) IL2R P01589 acute graft- D89.8 antecedent plasma 22927351 versus-host ; disease (aGVHD) serum IL2R P01589 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum IL2R P01589 acute graft- D89.8 monitoring plasma 22927351 versus-host ; disease (aGVHD) serum IL2R P01589 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum IL2R P01589 acute graft- D89.8 prognostic plasma 22927351 versus-host ; disease (aGVHD) serum IL-2Ra P01589 acute graft- D89.8 prognostic plasma 23165480 versus-host disease (aGVHD) IL-2Ra P01589 acute graft- D89.8 diagnostic plasma 23165480 versus-host disease (aGVHD) IL-6 P05231 graft-versus-host D89.8 prognostic plasma 23165480 disease (GVHD) IL-8 P10145 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum IL-8 P10145 acute graft- D89.8 prognostic plasma 23165480 versus-host disease (aGVHD) IL-8 P10145 acute graft- D89.8 diagnostic plasma 23165480 versus-host disease (aGVHD) IL-8 P10145 graft-versus-host D89.8 prognostic plasma 23165480 disease (GVHD) IL-8 P10145 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum M-CSF P09603 acute graft- D89.8 monitoring plasma 22927351 versus-host ; disease (aGVHD) serum M-CSF P09603 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum M-CSF P09603 acute graft- D89.8 antecedent plasma 22927351 versus-host ; disease (aGVHD) serum REG3a Q06141 graft-versus-host D89.8 differentiati plasma 23165480 disease (GVHD) on REG3a Q06141 graft-versus-host D89.8 prognostic plasma 23165480 disease (GVHD) REG3a Q06141 GI GVHD D89.8 diagnostic plasma 23165480 REG3a Q06141 graft-versus-host D89.8 prognostic; plasma 23165480 disease (GVHD) theragnostic REG3a Q06141 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum REG3a Q06141 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum sc-kit P10721 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum sc-kit P10721 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum sFas acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum sFas acute graft- D89.8 antecedent plasma 22927351 versus-host ; disease (aGVHD) serum sICAM-1 P05362 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum Syndecan-1 P18827 acute graft- D89.8 diagnostic plasma 23165480 versus-host disease (aGVHD) Syndecan-I P18827 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum Syndecan-I P18827 acute graft- D89.8 monitoring plasma 22927351 versus-host ; disease (aGVHD) serum TGF-β1 P01137 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum TGF-β1 P01137 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum TNF-alpha P01375 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum TNF-alpha P01375 graft-versus-host D89.8 prognostic plasma 23165480 disease (GVHD) TNF-alpha P01375 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum TNFR1 P19438 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum TNFR1 P19438 acute graft- D89.8 monitoring plasma 22927351 versus-host ; disease (aGVHD) serum TNFR1 P19438 acute graft- D89.8 diagnostic plasma 23165480 versus-host disease (aGVHD) TNFR1 P19438 graft-versus-host D89.8 prognostic plasma 23165480 disease (GVHD) TNFR1 P19438 graft-versus-host D89.8 prognostic plasma 23165480 disease (GVHD) TNFR1 P19438 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum TNFR1 P19438 acute graft- D89.8 antecedent plasma 22927351 versus-host ; disease (aGVHD) serum TNFR1 P19438 acute graft- D89.8 prognostic plasma 22927351 versus-host ; disease (aGVHD) serum TNFR2 Q12933 acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum TNFR2 Q12933 acute graft- D89.8 monitoring plasma 22927351 versus-host ; disease (aGVHD) serum acute graft- D89.8 diagnostic plasma 22927351 versus-host ; disease (aGVHD) serum Adiponectin Q15848 Diabetic E10.2, diagnostic urine; 22698077 Nephropathy E11.2, serum E12.2, E13.2, E14.2 fibroblast growth Q9GZV9 Diabetic E10.2, prognostic serum 22698077 factor (FGF-23) Nephropathy E11.2, E12.2, E13.2, E14.2 a-Fetoprotein (AFP) P02771 testicular non- E29.9 Clinical classificatio serum 21037873 seminoma Society n Recom mended human chorionic P01215,P01233 testicular non- E29.9 Clinical classificatio serum 21037873 gonadotrophin (hCG) seminoma Society n Recom mended LDH P07864 testicular non- E29.9 Clinical classificatio serum 21037873 seminoma Society n Recom mended glycosaminoglycans mucopolysacchari E76 antecedent serum; 22658917 doses (MPSs) plasma glycosaminoglycans mucopolysacchari E76 diagnostic serum; 22658917 doses (MPSs) plasma glycosaminoglycans mucopolysacchari E76 prognostic; serum; 22658917 doses (MPSs) theragnostic plasma oligosaccharides mucopolysacchari E76 diagnostic urine; 22658917 doses (MPSs) plasma oligosaccharides mucopolysacchari E76 prognostic; urine; 22658917 doses (MPSs) theragnostic plasma oligosaccharides mucopolysacchari E76 prognostic urine; 22658917 doses (MPSs) plasma Bis(monoacylglycero)p mucopolysacchari E76 antecedent 22658917 hosphate doses (MPSs) Ganglioside 2 (GM2) mucopolysacchari E76 prognostic; 22658917 doses (MPSs) theragnostic Ganglioside 2 (GM2) mucopolysacchari E76 prognostic 22658917 doses (MPSs) Ganglioside 3 (GM3) mucopolysacchari E76 prognostic; 22658917 doses (MPSs) theragnostic Ganglioside 3 (GM3) mucopolysacchari E76 prognostic 22658917 doses (MPSs) prolactin P01236 Schizophrenia F20 pharmacody plasma 23129338 namic; theragnostic amyloid peptide schizophrenia F20 classificatio 23129339 n glucose schizophrenia F20 monitoring; blood 23129340 theragnostic lipid schizophrenia F20 monitoring; blood 23129340 theragnostic brain derived P23560 Huntington''s G10, F02.2 prognostic serum 21882408 neurotrophic factor Disease (BDNF) C-reactive protein P02741 Huntington''s G10, F02.2 marker plasma 21882408 (CRP) Disease CC‐chemokine ligand P51671 amyotrophic G12.2 diagnostic CSF; 21989244 11 (CCL11) lateral sclerosis blood (ALS) CCL24 O00175 amyotrophic G12.2 diagnostic CSF; 21989244 lateral sclerosis blood (ALS) CCL26 Q9Y258 amyotrophic G12.2 diagnostic CSF; 21989244 lateral sclerosis blood (ALS) Complement factors P01024 amyotrophic G12.2 diagnostic blood 21989244 C3 lateral sclerosis (ALS) IL-8 P10145 amyotrophic G12.2 diagnostic CSF; 21989244 lateral sclerosis blood (ALS) monocyte P13500 amyotrophic G12.2 diagnostic CSF; 21989244 chemoattractant lateral sclerosis blood protein 1 (MCP-1) (ALS) phosphorylated P12036 amyotrophic G12.2 diagnostic CSF; 21989244 neurofilament heavy lateral sclerosis blood chain (pNfH) (ALS) 102 plasma proteins Q969N4 Parkinson''s G20, F02.3 monitoring plasma 22814541 disease Ceruloplasmin P00450 Parkinson''s G20, F02.3 associative blood 23587062 disease High sensitivity C- P02741 Parkinson''s G20, F02.3 associative blood 23587062 reactive protein (Hs- disease CRP) IL-6 P05231 Parkinson''s G20, F02.3 associative blood 23587062 disease S100B P04271 Parkinson''s G20, F02.3 associative blood 23587062 disease Arginine Parkinson''s G20, F02.3 associative blood 23587062 disease cholesterol Parkinson''s G20, F02.3 associative blood 23587062 disease Coenzyme Q10 Parkinson''s G20, F02.3 associative blood 23587062 disease Copper Parkinson''s G20, F02.3 associative blood 23587062 disease F2 isoprostanes Parkinson''s G20, F02.3 associative blood 23587062 disease glycine Parkinson''s G20, F02.3 associative blood 23587062 disease Homocysteine Parkinson''s G20, F02.3 associative blood 23587062 disease Hydroxyeicosatetraen Parkinson''s G20, F02.3 associative blood 23587062 oic acid products disease (HETEs) Leucocyte 8-hydroxy- Parkinson''s G20, F02.3 associative blood 23587062 2-deoxygyanosine (8- disease OHdG) levodopa Parkinson''s G20, F02.3 associative blood 23587062 disease Malondialdehyde Parkinson''s G20, F02.3 associative blood 23587062 (MDA) disease Nitrate Parkinson''s G20, F02.3 associative blood 23587062 disease Non-Ceruloplasmin Parkinson''s G20, F02.3 associative blood 23587062 bound Copper (NCBC) disease HMPAO Parkinson''s G20, F02.3 associative 23587062 disease N-isopropyl-P[123I]- Parkinson''s G20, F02.3 associative 23587062 iodoamphetamine disease ([123I]IMP) chemokine C–C motif P13500 Alzheimer''s G30, F00 diagnostic CSF; 23631871 ligand 2 (CCL2; disease plasma monocyte chemotactic protein- 1; MCP-1 ) chemokine O00590 Frontotemporal G31.0 diagnostic CSF; 22527778 lobar blood degeneration (FTLD) cytokines Frontotemporal G31.0 diagnostic CSF; 22527778 lobar blood degeneration (FTLD) albumin (ALB) P43652 neuromyelitis G36.0 diagnostic serum 22570066 optica (NMO) IgG neuromyelitis G36.0 diagnostic serum 22570066 optica (NMO) NMO-IgG neuromyelitis G36.0 diagnostic serum 22570066 optica (NMO) sTNF-R1 P19438,P20333 Obstructive sleep G47.3 diagnostic plasma 22732129 apnea dipeptidyl peptidase P27487 chronic fatigue G93.3 diagnostic plasma 22732129 IV syndrome/myalgi c encephalomyelitis (CFS/ME) IFNγ P01579 chronic fatigue G93.3 diagnostic plasma 22732129 syndrome/myalgi c encephalomyelitis (CFS/ME) IL-12 Q99665 chronic fatigue G93.3 diagnostic plasma 22732129 syndrome/myalgi c encephalomyelitis (CFS/ME) IL-13 P35225 chronic fatigue G93.3 diagnostic plasma 22732129 syndrome/myalgi c encephalomyelitis (CFS/ME) IL-15 P40933 chronic fatigue G93.3 diagnostic plasma 22732129 syndrome/myalgi c encephalomyelitis (CFS/ME) IL-17 Q16552 chronic fatigue G93.3 diagnostic plasma 22732129 syndrome/myalgi c encephalomyelitis (CFS/ME) IL-1a P01583 chronic fatigue G93.3 diagnostic plasma 22732129 syndrome/myalgi c encephalomyelitis (CFS/ME) IL-1β P01584 chronic fatigue G93.3 diagnostic plasma 22732129 syndrome/myalgi c encephalomyelitis (CFS/ME) IL-2 P60568 chronic fatigue G93.3 diagnostic plasma 22732129 syndrome/myalgi c encephalomyelitis (CFS/ME) IL-23 Q9NPF7 chronic fatigue G93.3 diagnostic plasma 22732129 syndrome/myalgi c encephalomyelitis (CFS/ME) IL-4 P05112 chronic fatigue G93.3 diagnostic plasma 22732129 syndrome/myalgi c encephalomyelitis (CFS/ME) IL-5 P05113 chronic fatigue G93.3 diagnostic plasma 22732129 syndrome/myalgi c encephalomyelitis (CFS/ME) IL-6 P05231 chronic fatigue G93.3 diagnostic plasma 22732129 syndrome/myalgi c encephalomyelitis (CFS/ME) IL-8 P10145 chronic fatigue G93.3 diagnostic plasma 22732129 syndrome/myalgi c encephalomyelitis (CFS/ME) Lymphotoxin alpha P01374 chronic fatigue G93.3 diagnostic plasma 22732129 (LTα) syndrome/myalgi c encephalomyelitis (CFS/ME) TNF-alpha P01375 chronic fatigue G93.3 diagnostic plasma 22732129 syndrome/myalgi c encephalomyelitis (CFS/ME) vitamin E chronic fatigue G93.3 diagnostic serum 22732129 syndrome/myalgi c encephalomyelitis (CFS/ME) 8-iso-prostaglandin chronic fatigue G93.3 diagnostic 22732129 F(2 alpha) isoprostane syndrome/myalgi c encephalomyelitis (CFS/ME) cortisol chronic fatigue G93.3 prognostic; 22732129 syndrome/myalgi theragnostic c encephalomyelitis (CFS/ME) nitric oxide (NO) glaucoma H40-H42 marker 22827637 atrial natriuretic cardiac disease in I00-I99 diagnostic 22797141 peptide (ANP) infants B-type natriuretic Cardiovascular I00-I99 prognostic 22489717 peptide (BNP) disease (CVD) in patients with chronic kidney disease (CKD) B-type natriuretic cardiovascular I00-I99 antecedent 22681965 peptide (BNP) disease calcium Cardiovascular I00-I99 prognostic 22489717 disease (CVD) in patients with chronic kidney disease (CKD) Creatinine Cardiovascular I00-I99 prognostic 22489717 disease (CVD) in patients with chronic kidney disease (CKD) Homocysteine Cardiovascular I00-I99 prognostic 22489717 disease (CVD) in patients with chronic kidney disease (CKD) N-terminal pro-B-type Cardiovascular I00-I99 prognostic 22489717 natriuretic peptide disease (CVD) in (NT-pro-BNP) patients with chronic kidney disease (CKD) N-terminal pro-B-type critically ill cardiac I00-I99 prognostic 21440204 natriuretic peptide patient (NT-pro-BNP) parathyroid hormone Cardiovascular I00-I99 prognostic 22489717 (iPTH) disease (CVD) in patients with chronic kidney disease (CKD) Vitamin D Cardiovascular I00-I99 prognostic 22489717 disease (CVD) in patients with chronic kidney disease (CKD) B-type natriuretic acute coronary I20.0 prognostic 23331845 peptide (BNP) syndrome (ACS) N-terminal pro-B-type acute coronary I20.0 prognostic 23331845 natriuretic peptide syndrome (ACS) (NT-pro-BNP) B-type natriuretic Myocardial I21,I22 diagnostic 23331845 peptide (BNP) infarction N-terminal pro-B-type Myocardial I21,I22 diagnostic 23331845 natriuretic peptide infarction (NT-pro-BNP) adrenomedullin acute myocardial I21,I22,I50 prognostic 21440204 infarction; heart failure MR- acute myocardial I21,I22,I50 prognostic 21440204 proadrenomedullin infarction; heart failure Myeloid-related P06702 acute myocardial I21-I22 diagnostic plasma 23068427 protein-14 (MIM infarction 123886 S100A9, Mrp- 14) acute myocardial I21-I22 diagnostic plasma 23068427 infarction B-type natriuretic pulmonary I26 prognostic 21440204 peptide (BNP) embolism N-terminal pro-B-type pulmonary I26 prognostic 21440204 natriuretic peptide embolism (NT-pro-BNP) N-terminal pro-B-type Pulmonary I27.0, I27.2 diagnostic serum 22988462 natriuretic peptide hypertension in (NT-pro-BNP) Systemic sclerosis (SSc-PH) brain natriuretic Pulmonary I27.0, I27.2 diagnostic serum 22988462 peptide (BNP) hypertension in Systemic sclerosis (SSc-PH) B-type natriuretic pulmonary artery I27.0, I27.2 prognostic 21440204 peptide (BNP) hypertension N-terminal pro-B-type pulmonary artery I27.0, I27.2 prognostic 21440204 natriuretic peptide hypertension (NT-pro-BNP) Fetuin A P02765 Calcific Aortic I35 prognostic serum 22489716 Valve Disease Osteopontin (OPN) P10451 Calcific Aortic I35 monitoring plasma 22489716 Valve Disease lipid Calcific Aortic I35 prognostic 22489716 Valve Disease Galectin-3 P17931 Heart Failure (HF) I50 FDA prognostic plasma 22980054 approve d hs-C-reactive protein P02741 Heart Failure (HF) I50 prognostic serum 22980054 neutrophil gelatinase- P80188 Heart Failure (HF) I50 FDA prognostic serum 22980054 associated lipocalin approve (NGAL) d homocysteine Heart Failure (HF) I50 prognostic serum 22980054 B-type natriuretic heart failure I50 FDA prognostic 22489715 peptide (BNP) approve d B-type natriuretic heart failure I50 diagnostic; 22681965 peptide (BNP) prognostic N-terminal brain I50 clinical prognostic 19176440 natriuretic peptide trial (BNP) NT-pro-ANP heart failure I50 clinical prognostic 22489715 trial N-terminal Pro-BNP acute congestive I50 FDA diagnostic 22980054 (NP) HF (CHF) approve d Aldosterone heart failure I50 prognostic 22489715 Arginine vasopressin heart failure I50 prognostic 22489715 (AVP) F2 isoprostanes heart failure I50 prognostic 22489715 Norepinephrine heart failure I50 prognostic 22489715 N-terminal Pro-BNP Heart Failure (HF) I50 prognostic 22980054 (NP) N-terminal Pro-BNP Heart Failure (HF) I50 antecedent 22980054 (NP) N-terminal Pro-BNP Heart Failure (HF) I50 diagnostic 22980054 (NP) MR-pro-ANP heart failure I50 clinical prognostic 22489715 trial brain-type natriuretic I50 phase IV prognostic 23821090 peptide (BNP) MR- heart failure I50 prognostic 22489715 proadrenomedullin natriuretic peptide heart failure (HF) I50 prognostic 23143510 tissue inhibitor of heart failure I50 prognostic 22489715 metalloproteinase (TIMP 1) brain natriuretic Heart Failure (HF) I50 diagnostic 22980054 peptide (BNP) brain natriuretic Heart Failure (HF) I50 prognostic 22980054 peptide (BNP) C-terminal pro- heart failure I50 prognostic 22489715 endothelin-1 microRNA (which acute coronary I50,I10,I11,I diagnostic plasma 22824111 microRNA?) syndrome,acute 12,I13,I15,E myocardial 11,I21,I22,I2 infarction, type 2 0.0 diabetes, hypertension, heart failure Cathepsin S P25774 Premature I70 diagnostic; plasma 22489712 Atherosclerosis antecedent Cystatin C P01034 Premature I70 diagnostic; plasma 22489712 Atherosclerosis antecedent Lipoprotein-associated Q13093 Premature I70 diagnostic; serum 22489712 phospholipase A2 (Lp- Atherosclerosis antecedent PLA2) Myeloid-related P06702 carotid artery I70 antecedent plasma 23068427 protein-14 (MIM atherosclerosis 123886 S100A9, Mrp- 14) Myeloid-related P05109 carotid artery I70 antecedent plasma 23068427 protein-8 (MIM atherosclerosis 123885 S100A8, Mrp- 8) B-type natriuretic Atherosclerosis I70 diagnostic 22577298 peptide (BNP) 3-Nitrotyrosine (3-NT) carotid artery I70 diagnostic 23068427 atherosclerosis F2 isoprostanes Atherosclerosis I70 diagnostic 22577298 homocysteine Premature I70 diagnostic; 22489712 Atherosclerosis antecedent Asymmetric Atherosclerosis I70 diagnostic 22577298 dimethylarginine (ADMA) Asymmetric Atherosclerosis I70 prognostic 22577298 dimethylarginine (ADMA) adhesion molecule-1 Peripheral arterial I73 antecedent 22489720 disease (PAD) apolipoprotein A P06727,P02647 Atherothrombosis I80-I82 antecedent blood 23630624 (ApoA) ,P02652,Q6Q7 88 apolipoprotein B Q0VD83 Atherothrombosis I80-I82 antecedent blood 23630624 (ApoB) cardiac troponin I P19429 Atherothrombosis I80-I82 used in antecedent blood 23630624 (cTnI) clinic cardiac troponin T P45379 Atherothrombosis I80-I82 used in antecedent blood 23630624 (cTnT) clinic C-reactive protein P02741 Atherothrombosis I80-I82 used in antecedent blood 23630624 (CRP) clinic cystatin C P01034 Atherothrombosis I80-I82 antecedent plasma 23630624 fibrinogen P02679,P02671 Atherothrombosis I80-I82 used in antecedent blood 23630624 ,P02675 clinic fibrinogen P02679,P02671 Atherothrombosis I80-I82 antecedent blood 23630624 ,P02675 high density Q00341 Atherothrombosis I80-I82 antecedent serum 23630624 lipoprotein (HDL) high sensitivity C- P02741 Atherothrombosis I80-I82 used in antecedent blood 23630624 reactive protein (hs clinic CRP) cholesterol Atherothrombosis I80-I82 used in antecedent blood 23630624 clinic Creatinine Atherothrombosis I80-I82 antecedent serum 23630624 uric acid Atherothrombosis I80-I82 antecedent serum 23630624 Vitamin D Atherothrombosis I80-I82 antecedent serum 23630624 D-dimer venous I80-I82 prognostic 22528325 thromboembolis m (VTE) D-dimer deep venous I82.4 antecedent 22528325 thrombosis (DVT) D-dimer deep venous I82.4 prognostic 22528325 thrombosis (DVT) MR-pro-ANP lower respiratory J09-J99 classificatio 21440204 tract infections n Pro-ANP lower respiratory J09-J99 classificatio 21440204 tract infections n MR- community- J12, J13, classificatio 21440204 proadrenomedullin acquired J14, J15, n pneumonia J16, J17, J18, P23 Aa-Val360 Chronic J40-J44, J47 diagnostic plasma 23361193 obstructive pulmonary disease (COPD) C-reactive protein P02741 Chronic J40-J44, J47 diagnostic plasma 23361193 (CRP) obstructive pulmonary disease (COPD) fibrinogen P02679,P02671 Chronic J40-J44, J47 prognostic plasma 23361193 ,P02675 obstructive pulmonary disease (COPD) immunoglobulin light- Chronic J40-J44, J47 diagnostic plasma 23361193 chain obstructive pulmonary disease (COPD) PARC/CCL18 P55774 Chronic J40-J44, J47 diagnostic plasma 23361193 obstructive pulmonary disease (COPD) PARC/CCL18 P55774 Chronic J40-J44, J47 prognostic plasma 23361193 obstructive pulmonary disease (COPD) desmosine Chronic J40-J44, J47 diagnostic urine; 23361193 obstructive plasma pulmonary disease (COPD) b-defensins Chronic J40-J44, J47 diagnostic 23361193 obstructive pulmonary disease (COPD) brain natriuretic chronic lung J40- prognostic serum 22988462 peptide (BNP) disease J44,J45,J47, P27.1 Aa-Val360 emphysema J43 prognostic plasma 23361193 high-sensitivity C- P02741 asthma J45 diagnostic serum; 22796631 reactive protein (hs- EBC CRP) YKL-40 P36222 asthma J45 diagnostic serum 19532094 YKL-40 P36222 asthma J45 monitoring serum 19532094 exhaled nitric oxide asthma J45 diagnostic 22877617 (eNO) pro-ANP acute lung injury J80 prognostic 21440204 CC Chemokine Ligand P55774 Systemic sclerosis J80,J81,J82,J prognostic serum 22988462 18 (CCL18) associated 84,M34 interstitial lung disease (SSc-ILD) CC Chemokine Ligand O00590 Systemic sclerosis J80,J81,J82,J diagnostic serum; 22988462 2 (CCL2 associated 84,M34 BAL interstitial lung disease (SSc-ILD) connective tissue P29279 Systemic sclerosis J80,J81,J82,J diagnostic serum 22988462 growth factor (CTGF) associated 84,M34 interstitial lung disease (SSc-ILD) CXC Chemokine Ligand P02778 Systemic sclerosis J80,J81,J82,J diagnostic serum 22988462 10 (CXCL10) associated 84,M34 interstitial lung disease (SSc-ILD) IL-10 P22301 Systemic sclerosis J80,J81,J82,J diagnostic serum 22988462 associated 84,M34 interstitial lung disease (SSc-ILD) IL-13 P35225 Systemic sclerosis J80,J81,J82,J diagnostic serum 22988462 associated 84,M34 interstitial lung disease (SSc-ILD) IL-17 Q16552 Systemic sclerosis J80,J81,J82,J diagnostic serum 22988462 associated 84,M34 interstitial lung disease (SSc-ILD) IL-22 Q9GZX6 Systemic sclerosis J80,J81,J82,J diagnostic serum 22988462 associated 84,M34 interstitial lung disease (SSc-ILD) IL-4 P05112 Systemic sclerosis J80,J81,J82,J diagnostic serum 22988462 associated 84,M34 interstitial lung disease (SSc-ILD) IL-6 P05231 Systemic sclerosis J80,J81,J82,J diagnostic serum 22988462 associated 84,M34 interstitial lung disease (SSc-ILD) Krebs Von Den Lungen P15941 Systemic sclerosis J80,J81,J82,J diagnostic serum 22988462 6 (KL-6) associated 84,M34 interstitial lung disease (SSc-ILD) Krebs Von Den Lungen P15941 Systemic sclerosis J80,J81,J82,J monitoring; serum 22988462 6 (KL-6) associated 84,M34 theragnostic interstitial lung disease (SSc-ILD) Krebs Von Den Lungen P15941 Systemic sclerosis J80,J81,J82,J prognostic serum 22988462 6 (KL-6) associated 84,M34 interstitial lung disease (SSc-ILD) Matrix P09237 Systemic sclerosis J80,J81,J82,J diagnostic serum 22988462 Metalloproteinase 7 associated 84,M34 (MMP7) interstitial lung disease (SSc-ILD) matrix P14780 Systemic sclerosis J80,J81,J82,J diagnostic serum; 22988462 metalloproteinase 9 associated 84,M34 BAL (MMP 9) interstitial lung disease (SSc-ILD) monocyte P13500 Systemic sclerosis J80,J81,J82,J diagnostic serum; 22988462 chemoattractant associated 84,M34 BAL protein 1 (MCP-1) interstitial lung disease (SSc-ILD) Surfactant Protein A Q8IWL2,Q8IWL Systemic sclerosis J80,J81,J82,J diagnostic serum 22988462 (SP-A) 1 associated 84,M34 interstitial lung disease (SSc-ILD) Surfactant Protein D P35247 Systemic sclerosis J80,J81,J82,J diagnostic serum 22988462 (SP-D) associated 84,M34 interstitial lung disease (SSc-ILD) isoprostane Systemic sclerosis J80,J81,J82,J diagnostic serum 22988462 associated 84,M34 interstitial lung disease (SSc-ILD) albumin (ALB) P43652 idiopathic J84.1 prognostic serum 22424426 pulmonary fibrosis (IPF) CCL18 P55774 idiopathic J84.1 monitoring blood 22424426 pulmonary fibrosis (IPF) CCL18 P55774 idiopathic J84.1 prognostic blood 22424426 pulmonary fibrosis (IPF) CD28 P10747 idiopathic J84.1 monitoring blood 22424426 pulmonary fibrosis (IPF) CD28 P10747 idiopathic J84.1 prognostic blood 22424426 pulmonary fibrosis (IPF) CD4 P01730 idiopathic J84.1 monitoring blood 22424426 pulmonary fibrosis (IPF) CD4 P01730 idiopathic J84.1 prognostic blood 22424426 pulmonary fibrosis (IPF) Krebs Von Den Lungen P15941 idiopathic J84.1 prognostic blood 22424426 6 (KL-6) pulmonary fibrosis (IPF) Krebs Von Den Lungen P15941 idiopathic J84.1 differentiati blood 22424426 6 (KL-6) pulmonary on fibrosis (IPF) Matrix P09237 idiopathic J84.1 differentiati blood 22424426 Metalloproteinase 7 pulmonary on (MMP7) fibrosis (IPF) Matrix P09237 idiopathic J84.1 prognostic blood 22424426 Metalloproteinase 7 pulmonary (MMP7) fibrosis (IPF) Matrix P09237 idiopathic J84.1 monitoring blood 22424426 Metalloproteinase 7 pulmonary (MMP7) fibrosis (IPF) MMP1 P03956 idiopathic J84.1 differentiati blood 22424426 pulmonary on fibrosis (IPF) Osteopontin (OPN) P10451 idiopathic J84.1 monitoring blood 22424426 pulmonary fibrosis (IPF) Periostin Q15063 idiopathic J84.1 differentiati blood 22424426 pulmonary on fibrosis (IPF) Periostin Q15063 idiopathic J84.1 prognostic blood 22424426 pulmonary fibrosis (IPF) SP-D P35247 idiopathic J84.1 differentiati blood 22424426 pulmonary on fibrosis (IPF) SP-D P35247 idiopathic J84.1 prognostic blood 22424426 pulmonary fibrosis (IPF) Surfactant Protein A Q8IWL2,Q8IWL idiopathic J84.1 prognostic blood 22424426 (SP-A) 1 pulmonary fibrosis (IPF) Surfactant Protein A Q8IWL2,Q8IWL idiopathic J84.1 differentiati blood 22424426 (SP-A) 1 pulmonary on fibrosis (IPF) vascular endothelial P15692,P49765 idiopathic J84.1 prognostic blood 22424426 growth factor (VEGF) ,P49767,O4391 pulmonary 5 fibrosis (IPF) YKL-40 P36222 idiopathic J84.1 prognostic blood 22424426 pulmonary fibrosis (IPF) YKL-40 P36222 idiopathic J84.1 monitoring blood 22424426 pulmonary fibrosis (IPF) Brain natriuretic idiopathic J84.1 prognostic serum 22424426 peptide (BNP) pulmonary fibrosis (IPF) CYFRA 21-1 malignant pleural J91.0 diagnostic serum; 8 effusion pleural effusio n eosinophil-derived P10153 Eosinophilic K20.0 monitoring tissue; 22920068 neurotoxin (EDN) esophagitis (EoE) serum IL-13 P35225 Eosinophilic K20.0 surrogate tissue; 22920068 esophagitis (EoE) serum kynurenine Crohn''s disease K50 monitoring serum 22424434 (CD) tryptophan Crohn''s disease K50 monitoring serum 22424434 (CD) antibodies to Cbir1 Inflammatory K50,K51 prognostic serum 22424434 Bowel Disease (IBD) antibodies to Fla- X Inflammatory K50,K51 prognostic serum 22424434 Bowel Disease (IBD) antibodies to Fla- X Inflammatory K50,K51 diagnostic serum 22424434 Bowel Disease (IBD) antibodies to Fla- X Inflammatory K50,K51 differentiati serum 22424434 Bowel Disease on (IBD) antibodies to Flagellin Inflammatory K50,K51 prognostic serum 22424434 A4-Fla2 Bowel Disease (IBD) antibodies to Flagellin Inflammatory K50,K51 diagnostic serum 22424434 A4-Fla2 Bowel Disease (IBD) antibodies to Flagellin Inflammatory K50,K51 differentiati serum 22424434 A4-Fla2 Bowel Disease on (IBD) Antibodies to outer Inflammatory K50,K51 diagnostic serum 22424434 membrane porin Bowel Disease (Anti-OmpC) (IBD) Antibodies to outer Inflammatory K50,K51 differentiati serum 22424434 membrane porin Bowel Disease on (Anti-OmpC) (IBD) Antibodies to outer Inflammatory K50,K51 prognostic serum 22424434 membrane porin Bowel Disease (Anti-OmpC) (IBD) antichitobioside Inflammatory K50,K51 diagnostic serum 22424434 carbohydrate IgA Bowel Disease (ACCA) (IBD) antichitobioside Inflammatory K50,K51 differentiati serum 22424434 carbohydrate IgA Bowel Disease on (ACCA) (IBD) Anti-I2 Inflammatory K50,K51 prognostic serum 22424434 Bowel Disease (IBD) antilaminaribioside Inflammatory K50,K51 diagnostic serum 22424434 carbohydrate IgG Bowel Disease (ALCA) (IBD) antilaminaribioside Inflammatory K50,K51 differentiati serum 22424434 carbohydrate IgG Bowel Disease on (ALCA) (IBD) Anti-neutrophil Inflammatory K50,K51 diagnostic serum 22424434 cytoplasmic Bowel Disease antibodies (ANCAs) (IBD) Anti-neutrophil Inflammatory K50,K51 differentiati serum 22424434 cytoplasmic Bowel Disease on antibodies (ANCAs) (IBD) anti-Saccharomyces Inflammatory K50,K51 diagnostic serum 22424434 cerevisiae antibodies Bowel Disease (ASCA) (IBD) anti-Saccharomyces Inflammatory K50,K51 differentiati serum 22424434 cerevisiae antibodies Bowel Disease on (ASCA) (IBD) anti-Saccharomyces Inflammatory K50,K51 prognostic serum 22424434 cerevisiae antibodies Bowel Disease (ASCA) (IBD) anti-synthetic Inflammatory K50,K51 diagnostic serum 22424434 mannoside antibodies Bowel Disease (AΣMA or AMCA) (IBD) anti-synthetic Inflammatory K50,K51 differentiati serum 22424434 mannoside antibodies Bowel Disease on (AΣMA or AMCA) (IBD) Flagellin (Anti-Cbir1) Inflammatory K50,K51 diagnostic serum 22424434 Bowel Disease (IBD) Flagellin (Anti-Cbir1) Inflammatory K50,K51 differentiati serum 22424434 Bowel Disease on (IBD) Pseudomonas Inflammatory K50,K51 diagnostic serum 22424434 flourescens-associated Bowel Disease sequence I–2 (Anti-I2) (IBD) Pseudomonas Inflammatory K50,K51 differentiati serum 22424434 flourescens-associated Bowel Disease on sequence I–2 (Anti-I2) (IBD) L-arginine ulcerative colitis K51 diagnostic serum 22424434 (UC) CK-18 P05783 steatohepatitis K70.1, K76.0 diagnostic serum; 23227839 plasma alanine Q8TD30,P2429 nonalcoholic K76.0 diagnostic serum 23227839 aminotransferase 8 steatohepatitis (ALT) (NASH) aspartate P17174 nonalcoholic K76.0 diagnostic serum 23227839 aminotransferase steatohepatitis (AST) (NASH) CK-18 P05783 nonalcoholic K76.0 differentiati serum 23227839 steatohepatitis on (NASH) CK-18 P05783 non-alcoholic K76.0 diagnostic serum 22567408 steatohepatitis (NASH) C-reactive protein P02741 nonalcoholic K76.0 diagnostic serum 23227839 (CRP) steatohepatitis (NASH) FAS (CD95) P25445 nonalcoholic K76.0 diagnostic serum 23227839 steatohepatitis (NASH) IL-6 P05231 nonalcoholic K76.0 diagnostic serum 23227839 steatohepatitis (NASH) M-30 P05783 nonalcoholic K76.0 diagnostic serum 23227839 steatohepatitis (NASH) M-65 P05783 nonalcoholic K76.0 diagnostic serum 23227839 steatohepatitis (NASH) TNF-alpha P01375 nonalcoholic K76.0 diagnostic serum 23227839 steatohepatitis (NASH) Type IV collagen 7S P53420,P29400 nonalcoholic K76.0 prognostic serum 23227839 ,P02462,P0857 steatohepatitis 2,Q14031,Q019 (NASH) 55 uncleaved CK-18 P05783 non-alcoholic K76.0 differentiati serum 22567408 steatohepatitis on (NASH) Fasting glucose nonalcoholic K76.0 diagnostic serum 23227839 steatohepatitis (NASH) Hyaluronic acid nonalcoholic K76.0 diagnostic serum 23227839 steatohepatitis (NASH) N-terminal propeptide nonalcoholic K76.0 diagnostic serum 23227839 of type III collagen steatohepatitis (PIIINP) (NASH) IL-8 P10145 acute graft- K76.5,A40- diagnostic plasma 23165480 versus-host A41,D56,K7 disease (aGVHD); 0.3, K71.7, rejection; liver K74,T86,D8 fibrosis in 9.8 thalassemic patients; sepsis;veno- occlusive disease IL-2Ra P01589 acute graft- K76.5,A40- diagnostic plasma 23165480 versus-host A41,D89.8 disease (aGVHD);sepsis;ve no-occlusive disease IL-6 P05231 acute graft- K76.5,A40- diagnostic plasma 23165480 versus-host A41,D89.8 disease (aGVHD);sepsis;ve no-occlusive disease C-reactive protein P02741 acute graft- K76.5,D59.4 diagnostic plasma 23165480 (CRP) versus-host ,D89.8 disease (aGVHD);microan giopathic hemolytic anemia;veno- occlusive disease TNF-alpha P01375 acute graft- K76.5,D59.4 diagnostic plasma 23165480 versus-host ,D89.8 disease (aGVHD);microan giopathic hemolytic anemia;veno- occlusive disease IgA anti-DGP Celiac Disease K90.0 used in diagnostic serum 23388848 (antideaminated clinic forms of gliadin peptides antibodies) IgA anti-EMA Celiac Disease K90.0 used in diagnostic serum 23388848 (antiendomysium clinic antibody) IgA anti-tTG Celiac Disease K90.0 used in diagnostic serum 23388848 clinic Adiponectin Q15848 psoriasis L40 diagnostic serum 23532439 alpha 2 -antiplasmin P08697 psoriasis L40 diagnostic blood 23532439 C4 psoriasis L40 diagnostic blood 23532439 C-reactive protein P02741 psoriasis L40 diagnostic serum 23532439 (CRP) fibrinogen P02679,P02671 psoriasis L40 diagnostic blood 23532439 ,P02675 haptoglobin P00738 psoriasis L40 diagnostic serum 23532439 IFNγ P01579 psoriasis L40 diagnostic serum 23532439 IL-12 Q99665 psoriasis L40 diagnostic serum 23532439 IL-18 Q14116 psoriasis L40 diagnostic serum 23532439 IL-6 P05231 psoriasis L40 diagnostic serum 23532439 IL-6 P05231 psoriasis L40 differentiati serum 23532439 on IL-8 P10145 psoriasis L40 diagnostic serum 23532439 leptin P41159 psoriasis L40 diagnostic serum 23532439 lipocalin Q6UWW0 psoriasis L40 diagnostic serum 23532439 Plasminogen P00747 psoriasis L40 diagnostic blood 23532439 Protein C psoriasis L40 diagnostic blood 23532439 prothrombin P00734 psoriasis L40 diagnostic blood 23532439 fragments 1+2 P-selectin P16109 psoriasis L40 diagnostic serum 23532439 resistin Q9HD89 psoriasis L40 diagnostic serum 23532439 TNF P01375 psoriasis L40 diagnostic serum 23532439 8-hydroxy guanosine psoriasis L40 diagnostic blood 23532439 Fibrinopeptide A psoriasis L40 diagnostic blood 23532439 Bβ and D-dimer psoriasis L40 diagnostic blood 23532439 KOA1 knee M15-M19, marker serum 22842200 (TGLESGHGPGDS) osteoarthritis M47 cartilage glycoprotein P36222 osteoarthritis M15- diagnostic serum; 17538566 39 (YKL-40) M19,M47 Synovi al fluid cartilage glycoprotein P36222 osteoarthritis M15- pharmacody serum; 17538566 39 (YKL-40) M19,M47 namic; Synovi theragnostic al fluid cartilage oligomeric P49747 osteoarthritis M15- diagnostic; serum; 17538566 protein (COMP) M19,M47 prognostic Synovi al fluid cartilage oligomeric P49747 osteoarthritis M15- diagnostic serum; 17538566 protein (COMP) M19,M47 Synovi al fluid COL2-3/4C (long) P02458 osteoarthritis M15- prognostic urine; 17538566 epitope M19,M47 serum COL2-3/4C (long) P02458 osteoarthritis M15- pharmacody urine; 17538566 epitope M19,M47 namic; serum theragnostic COL2-3/4C (short) P02458 osteoarthritis M15- prognostic urine; 17538566 epitope M19,M47 serum COL2-3/4C (short) P02458 osteoarthritis M15- pharmacody urine; 17538566 epitope M19,M47 namic; serum theragnostic Follistatin-like protein Q12841 osteoarthritis M15- monitoring serum 22842200 1 (FSTL1) M19,M47 lumican P51884 osteoarthritis M15- diagnostic serum 22842200 M19,M47 matrix P50281,P45452 osteoarthritis M15- prognostic serum 17538566 metalloproteinase ,O75900,P5151 M19,M47 (MMP) 1,Q99542,P515 12,P03956,Q8N 119,Q9NRE1,O 60882,Q9H239, Q9ULZ9,P0825 4,P22894,P399 00,P08253,P14 780,Q9H306,P0 9238,P24347,Q 9NPA2,P09237, Q9Y5R2 matrix P50281,P45452 osteoarthritis M15- pharmacody serum 17538566 metalloproteinase ,O75900,P5151 M19,M47 namic; (MMP) 1,Q99542,P515 theragnostic 12,P03956,Q8N 119,Q9NRE1,O 60882,Q9H239, Q9ULZ9,P0825 4,P22894,P399 00,P08253,P14 780,Q9H306,P0 9238,P24347,Q 9NPA2,P09237, Q9Y5R2 matrix P50281,P45452 osteoarthritis M15- diagnostic serum 17538566 metalloproteinase ,O75900,P5151 M19,M47 (MMP) 1,Q99542,P515 12,P03956,Q8N 119,Q9NRE1,O 60882,Q9H239, Q9ULZ9,P0825 4,P22894,P399 00,P08253,P14 780,Q9H306,P0 9238,P24347,Q 9NPA2,P09237, Q9Y5R2 osteocalcin (OC) P02818 osteoarthritis M15- prognostic serum 17538566 M19,M47 tetranectin P05452 osteoarthritis M15- diagnostic serum 22842200 M19,M47 tissue inhibitor of P16035,P35625 osteoarthritis M15- diagnostic serum 17538566 matrix ,P01033 M19,M47 metalloproteinase (TIMP) tissue inhibitor of P16035,P35625 osteoarthritis M15- prognostic serum 17538566 matrix ,P01033 M19,M47 metalloproteinase (TIMP) von Willebrand factor P04275 osteoarthritis M15- diagnostic serum 22842200 M19,M47 Hyaluronic acid osteoarthritis M15- diagnostic serum 17538566 M19,M47 Hyaluronic acid osteoarthritis M15- prognostic serum 17538566 M19,M47 N-propeptide IIA of osteoarthritis M15- prognostic serum 17538566 collagen type II M19,M47 (PIIANP) C-propeptide of osteoarthritis M15- diagnostic; serum; 17538566 collagen type II (PIICP) M19,M47 prognostic Synovi al fluid N-terminal cross- osteoarthritis M15- diagnostic urine; 17538566 linked telopeptide of M19,M47 serum type I collagen (NTX-I) Pentosidine osteoarthritis M15- prognostic urine; 17538566 M19,M47 serum nine-amino-acid osteoarthritis M15- prognostic urine; 17538566 peptide of type II M19,M47 serum collagen (Coll 2-1) nitrated form of nine- osteoarthritis M15- prognostic urine; 17538566 amino-acid peptide of M19,M47 serum type II collagen (Coll 2- 1 NO2) C-terminal cross- osteoarthritis M15- prognostic urine; 17538566 linked telopeptide of M19,M47 serum type I collagen (CTX-I) Fibulin-3 peptide 1 osteoarthritis M15- diagnostic 22842200 M19,M47 Fibulin-3 peptide 2 osteoarthritis M15- diagnostic 22842200 M19,M47 N-propeptide II of osteoarthritis M15- diagnostic 17538566 collagen type II (PIINP) M19,M47 IFNα P01563,P01562 Systemic lupus M32 diagnostic serum 22732129 ,P32881,P0157 erythematosus 1,P05014,P015 68,P01567,P01 566,P01569,P0 1570,P05013,P 05015 endothelin-1 (ET-1) P05305 Systemic sclerosis M34 diagnostic serum 22988462 (SSc) soluble E-selectin (sE- P16581 Systemic sclerosis M34 diagnostic serum 22988462 selectin) (SSc) soluble vascular cell P19320 Systemic sclerosis M34 diagnostic serum 22988462 adhesion molecule- 1 (SSc) (sVCAM-1) vascular endothelial P15692,P49765 Systemic sclerosis M34 diagnostic serum 22988462 growth factor (VEGF) ,P49767,O4391 (SSc) 5 Haemopexin P02790 minimal change N00.0,N01.0 diagnostic serum; 23013941 nephropathy ,N02.0,N03. urine (MCN) 0,N04.0,N05 .0,N06.0,N0 7.0,N08.0 IL-13 P35225 minimal change N00.0,N01.0 prognostic Plasma 23013941 nephropathy ,N02.0,N03. ; (MCN) 0,N04.0,N05 lymph .0,N06.0,N0 ocytes 7.0,N08.0 sIL-2R P01589,P14784 minimal change N00.1,N01.1 diagnostic serum 23013941 ,P31785 nephropathy ,N02.1,N03. (MCN) ; Focal 1,N04.1,N05 segmental .1,N06.1,N0 glomerulonephriti 7.1,N08.1,N s (FSG) 00.0,N01.0, N02.0,N03.0 ,N04.0,N05. 0,N06.0,N07 .0,N08.0 soluble urokinase-type Q03405 Focal segmental N00-N08 diagnostic serum 23013941 plasminogen activator glomerulonephriti receptor (suPAR) s (FSG) anti aldose-reductase membranous N02.2 diagnostic serum 23013941 antibodies (Anti-AR nephropathy antibodies) (MN) anti-M type membranous N02.2 diagnostic serum 23013941 phospholipase A2 nephropathy receptor antibodies (MN) (Anti-PLA2R antibodies) anti-M type membranous N02.2 differentiati serum 23013941 phospholipase A2 nephropathy on receptor antibodies (MN) (Anti-PLA2R antibodies) Anti-SOD2 antibodies membranous N02.2 diagnostic serum 23013941 nephropathy (MN) Cystatin C P01034 ACUTE KIDNEY N17 diagnostic serum 22983082 INJURY (AKI) cystatin C (CyC) P01034 acute kidney N17 diagnostic urine;s 22731900 injury in pediatric erum cardiac patients IL-18 Q14116 acute kidney N17 diagnostic urine;s 22731900 injury in pediatric erum cardiac patients neutrophil gelatinase- P80188 acute kidney N17 clinical diagnostic urine;s 22731900 associated lipocalin injury in pediatric trial erum (NGAL) cardiac patients neutrophil gelatinase- P80188 ACUTE KIDNEY N17 diagnostic plasma 22983082 associated lipocalin INJURY (AKI) (NGAL) urea nitrogen ACUTE KIDNEY N17 diagnostic blood 22983082 INJURY (AKI) Creatinine ACUTE KIDNEY N17 diagnostic serum 22983082 INJURY (AKI) Creatinine kidney injury N17 diagnostic 22731900 ProBNP chronic kidney N18 prognostic 22914685 disease C-reactive protein P02741 overactive N32.81 diagnostic; serum 23314226 (CRP) bladder monitoring C-reactive protein P02741 overactive N32.81 prognostic serum 23314226 (CRP) bladder Nerve growth P01138 overactive N32.81 diagnostic; serum 23314226 factor(NGF) bladder monitoring Cystatin C P01034 renal alteration surrogate serum; 22716111 Glome rulus Eosinophil cationic P12724 allergic and diagnostic; serum 22683541 protein (ECP) eosinophilic monitoring diseases eosinophil peroxidase P11678 allergic and diagnostic; serum 22683541 (EPO) eosinophilic monitoring diseases eosinophil-derived P10153 allergic and diagnostic; serum 22683541 neurotoxin (EDN) eosinophilic monitoring diseases IL-6 P05231 risks of death and clinical prognostic serum 518 of SPC trial major basic protein P13727 allergic and diagnostic; serum 22683541 (MBP) eosinophilic monitoring diseases neutrophil gelatinase- P80188 renal alteration surrogate serum; 22716111 associated lipocalin Glome (NGAL) rulus Asymmetric CID501 pre-eclampsia O11, O14 monitoring serum 22943702 dimethylarginine (ADMA) Placental protein 13 pre-eclampsia O11, O14 clinical antecedent serum 22943702 (PP13) trial Pregnancy associated Q13219 pre-eclampsia O11, O14 clinical antecedent serum 22943702 plasma protein-A trial (PAPP-A) Pregnancy associated Q13219 pre-eclampsia O11, O14 monitoring serum 22943702 plasma protein-A (PAPP-A) soluble Fms-like P17948 pre-eclampsia O11, O14 monitoring serum 22943702 tyrosine kinase 1 (sFlt- 1) homocysteine pre-eclampsia O11, O14 monitoring serum 22943702 homocysteine pre-eclampsia O11, O14 diagnostic 22943702 Inhibin A P05111 subsequent O11.9 clinical antecedent serum 18771979 preeclampsia in trial patients with previous PE and/or chronic hypertension (CHTN) placenta growth P49763 subsequent O11.9 clinical antecedent serum 18771979 factor (PLGF) preeclampsia in trial patients with previous PE and/or chronic hypertension (CHTN) soluble fms-like P17948 subsequent O11.9 clinical antecedent serum 18771979 tyrosine kinase-1 (sFlt- preeclampsia in trial 1) patients with previous PE and/or chronic hypertension (CHTN) Angiopoietin 1 Q15389 bronchopulmonar P27.1 antecedent Cord 23523392 y dysplasia blood Clara cell secretory P17559 bronchopulmonar P27.1 antecedent Cord 23523392 protein y dysplasia blood; Infant blood Endostatin P39060 bronchopulmonar P27.1 antecedent Cord 23523392 y dysplasia blood Eosinophil cationic P12724 bronchopulmonar P27.1 antecedent Infant 23523392 protein (ECP) y dysplasia blood granulocyte colony Q99062 bronchopulmonar P27.1 antecedent Infant 23523392 stimulating factor (G- y dysplasia blood CSF) IFNγ P01579 bronchopulmonar P27.1 clinical antecedent Infant 23523392 y dysplasia trial blood IL-10 P22301 bronchopulmonar P27.1 clinical antecedent Infant 23523392 y dysplasia trial blood IL-17 Q16552 bronchopulmonar P27.1 antecedent Infant 23523392 y dysplasia blood IL-1β P01584 bronchopulmonar P27.1 antecedent Infant 23523392 y dysplasia blood IL-6 P05231 bronchopulmonar P27.1 clinical antecedent Infant 23523392 y dysplasia trial blood IL-8 P10145 bronchopulmonar P27.1 clinical antecedent Infant 23523392 y dysplasia trial blood Krebs Von Den Lungen P15941 bronchopulmonar P27.1 antecedent Cord 23523392 6 (KL-6) y dysplasia blood; Infant blood matrix P14780 bronchopulmonar P27.1 antecedent Cord 23523392 metalloproteinase 9 y dysplasia blood (MMP 9) monocyte P13500 bronchopulmonar P27.1 clinical antecedent Infant 23523392 chemoattractant y dysplasia trial blood protein 1 (MCP-1) placental growth P49763 bronchopulmonar P27.1 antecedent Cord 23523392 factor (PlGF) y dysplasia blood platelet derived P04085,P01127 bronchopulmonar P27.1 antecedent Infant 23523392 growth factor-BB y dysplasia blood isoform (PDGF-BB) soluble E-selectin P16581 bronchopulmonar P27.1 clinical antecedent Cord 23523392 y dysplasia trial blood; Infant blood soluble L-selectin P14151 bronchopulmonar P27.1 antecedent Infant 23523392 y dysplasia blood transforming growth P01137 bronchopulmonar P27.1 clinical antecedent Infant 23523392 factor beta 1 (TGFb1) y dysplasia trial blood tumor necrosis factor P01374 bronchopulmonar P27.1 antecedent Infant 23523392 beta (TNFb) y dysplasia blood type IV collagen (C-IV) P53420,P29400 bronchopulmonar P27.1 antecedent Infant 23523392 ,P02462,P0857 y dysplasia blood 2,Q14031,Q019 55 vascular endothelial P15692,P49765 bronchopulmonar P27.1 clinical antecedent Infant 23523392 growth factor (VEGF) ,P49767,O4391 y dysplasia trial blood 5 tissue inhibitor of bronchopulmonar P27.1 antecedent Cord 23523392 metalloproteinase y dysplasia blood (TIMP 1) B-type natriuretic persistent P29.3 diagnostic 22797141 peptide (BNP) pulmonary hypertension of the newborn N-terminal pro-B-type persistent P29.3 diagnostic 22797141 natriuretic peptide pulmonary (NT-pro-BNP) hypertension of the newborn B-type natriuretic patent ductus Q25.0 diagnostic 22797141 peptide (BNP) arteriosus N-terminal pro-B-type patent ductus Q25.0 diagnostic 22797141 natriuretic peptide arteriosus (NT-pro-BNP) HDL-cholesterol Autosomal- Q61 prognostic serum 22846584 Dominant Polycystic Kidney Disease vascular endothelial P15692,P49765 Von Hippel- Q85.8 marker serum 262 growth factor (VEGF) ,P49767,O4391 Lindau disease 5 B-type natriuretic asphyxia R09.0, T71 prognostic 22797141 peptide (BNP) N-terminal pro-B-type asphyxia R09.0, T71 prognostic 22797141 natriuretic peptide (NT-pro-BNP) IL-1Rα P14778 Hypoxia R09.02 diagnostic plasma 22732129 IL-1β P01584 Hypoxia R09.02 diagnostic plasma 22732129 Osteopontin (OPN) P10451 tumor-related R09.02 clinical surrogate plasma 173 hypoxia trial plasminogen activator P05121 tumor-related R09.02 clinical surrogate plasma 173 inhibitor 1 (PAI-1) hypoxia trial vascular endothelial P15692,P49765 tumor-related R09.02 clinical surrogate plasma 173 growth factor (VEGF) ,P49767,O4391 hypoxia trial 5 calcium hemodialysis R88.0 clinical marker serum 20650654 trial phosphorus hemodialysis R88.0 clinical marker serum 20650654 trial potassium hemodialysis R88.0 clinical marker serum 20650654 trial IL-6 P05231 rejection of renal T86.1 diagnostic urine;b 22914685 transplants lood soluble CD30 P28908 rejection of renal T86.1 prognostic plasma 22914685 transplants