This journal is © The Owner Societies 2001
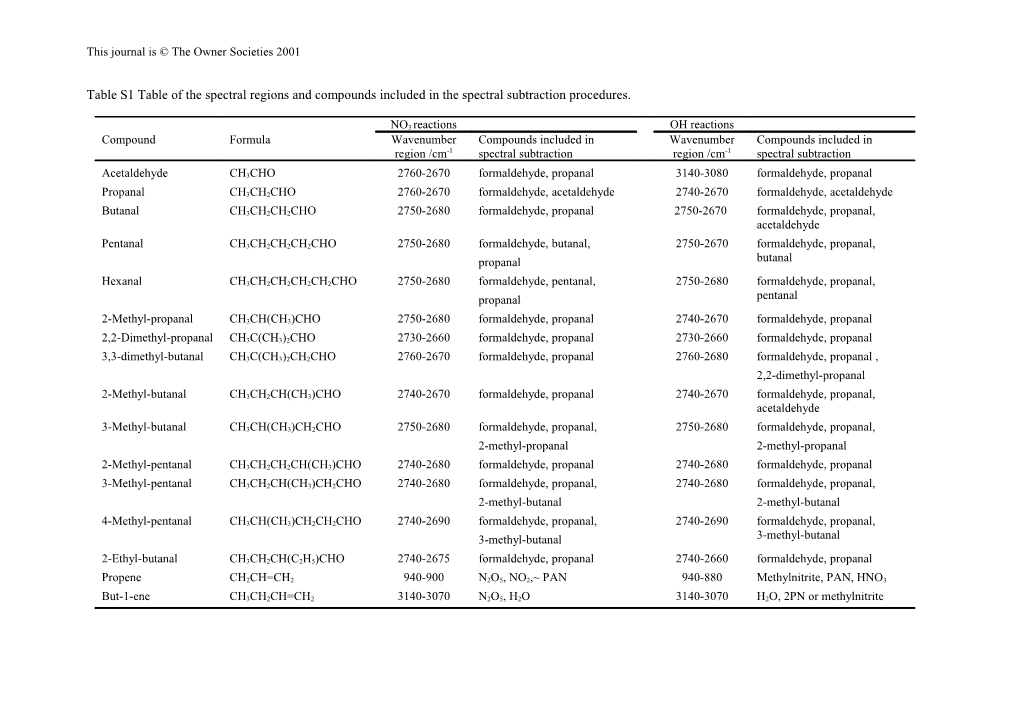
Table S1 Table of the spectral regions and compounds included in the spectral subtraction procedures.
NO3 reactions OH reactions Compound Formula Wavenumber Compounds included in Wavenumber Compounds included in region /cm-1 spectral subtraction region /cm-1 spectral subtraction
Acetaldehyde CH3CHO 2760-2670 formaldehyde, propanal 3140-3080 formaldehyde, propanal
Propanal CH3CH2CHO 2760-2670 formaldehyde, acetaldehyde 2740-2670 formaldehyde, acetaldehyde
Butanal CH3CH2CH2CHO 2750-2680 formaldehyde, propanal 2750-2670 formaldehyde, propanal, acetaldehyde
Pentanal CH3CH2CH2CH2CHO 2750-2680 formaldehyde, butanal, 2750-2670 formaldehyde, propanal, propanal butanal
Hexanal CH3CH2CH2CH2CH2CHO 2750-2680 formaldehyde, pentanal, 2750-2680 formaldehyde, propanal, propanal pentanal
2-Methyl-propanal CH3CH(CH3)CHO 2750-2680 formaldehyde, propanal 2740-2670 formaldehyde, propanal
2,2-Dimethyl-propanal CH3C(CH3)2CHO 2730-2660 formaldehyde, propanal 2730-2660 formaldehyde, propanal
3,3-dimethyl-butanal CH3C(CH3)2CH2CHO 2760-2670 formaldehyde, propanal 2760-2680 formaldehyde, propanal , 2,2-dimethyl-propanal
2-Methyl-butanal CH3CH2CH(CH3)CHO 2740-2670 formaldehyde, propanal 2740-2670 formaldehyde, propanal, acetaldehyde
3-Methyl-butanal CH3CH(CH3)CH2CHO 2750-2680 formaldehyde, propanal, 2750-2680 formaldehyde, propanal, 2-methyl-propanal 2-methyl-propanal
2-Methyl-pentanal CH3CH2CH2CH(CH3)CHO 2740-2680 formaldehyde, propanal 2740-2680 formaldehyde, propanal
3-Methyl-pentanal CH3CH2CH(CH3)CH2CHO 2740-2680 formaldehyde, propanal, 2740-2680 formaldehyde, propanal, 2-methyl-butanal 2-methyl-butanal
4-Methyl-pentanal CH3CH(CH3)CH2CH2CHO 2740-2690 formaldehyde, propanal, 2740-2690 formaldehyde, propanal, 3-methyl-butanal 3-methyl-butanal
2-Ethyl-butanal CH3CH2CH(C2H5)CHO 2740-2675 formaldehyde, propanal 2740-2660 formaldehyde, propanal
Propene CH2CH=CH2 940-900 N2O5, NO2,~ PAN 940-880 Methylnitrite, PAN, HNO3
But-1-ene CH3CH2CH=CH2 3140-3070 N2O5, H2O 3140-3070 H2O, 2PN or methylnitrite This journal is © The Owner Societies 2001
Figure S1. Plots of ln{[ald]0/[ald]t} vs. ln{[ref]0/[ref]t} for the decays of aldehydes and reference compounds during reaction with NO3. Different symbols indicate independent experiments. Errors quoted are the 3 statistical errors. (A) Acetaldehyde: 38 data points, five
experiments, krel = 0.194 0.021; (B) Propanal: 34 data points, four experiments, krel = 0.65
0.06; (C) Butanal: 49 data points, four experiments, krel = 0.91 0.08; (D) Pentanal: 41
data points, four experiments, krel = 1.27 0.16; (E) Hexanal: 41 data points, four
experiments, krel = 1.14 0.14; (F) 2-Methylpropanal: 37 data points, four experiments, krel
= 0.93 0.10; (G) 2,2-Dimethylpropanal: 31 data points, three experiments, krel = 1.92
0.16; (H) 2-Methylbutanal: 20 data points, two experiments, krel = 1.98 0.08; (I) 3-
Methylbutanal: 15 data points, two experiments, krel = 1.38 0.06; (L) 3,3–
Dimethylbutanal: 24 data points, two experiments, , krel = 1.27 0.10; (M) 2-
Methylpentanal: 19 data points, two experiments, krel = 1.99 0.08; (N) 3-Methylpentanal:
23 data points, two experiments, krel = 1.78 0.06; (O) 4-Methylpentanal: 23 data points,
three experiments, krel = 1.25 0.07; (P) 2-Ethylbutanal: 22 data points, two experiments,
krel = 3.31 0.07.
Figure S2. Plots of ln{[ald]0/[ald]t} vs. ln{[ref]0/[ref]t} for the decays of aldehydes and reference compounds during reaction with OH. Different symbols indicate independent experiments. Errors quoted are the 3 statistical errors. (A) Acetaldehyde: 19 data points, three
experiments, krel = 0.458 0.024; (B) Propanal: 23 data points, four experiments, krel = 0.72
0.06; (C) Butanal: 20 data points, three experiments, krel = 0.76 0.05; (D) Pentanal: 18
data points, three experiments, krel = 0.83 0.04; (E) Hexanal: 6 data points, one
experiment, krel = 0.91 0.04; (F) 2-Methylpropanal: 16 data points, two experiments, krel =
0.85 0.07; (G) 2,2-Dimethylpropanal: 26 data points, three experiments, krel = 0.85
0.04; (H) 2-Methylbutanal: 21 data points, three experiments, krel = 1.04 0.03; (I) 3-
Methylbutanal: 16 data points, two experiments, krel = 0.889 0.023; (L) 3,3 –
Dimethylbutanal: 28 data points, three experiments, krel = 0.68 0.05; (M) 2-
Methylpentanal: 16 data points, two experiments, krel = 1.06 0.07; (N) 3-Methylpentanal:
13 data points, two experiments, krel = 0.93 0.05; (O) 4-Methylpentanal: 13 data points,
two experiments, krel = 0.84 0.03; (P) 2-Ethylbutanal: 12 data points, two experiments, krel = 1.28 0.13. This journal is © The Owner Societies 2001
Figure S1.
0.6 1.5 } t } } ] t t 0.4 ] ] l l e a
A B a d n n C y a a t h p 1.2 e u o r d b l [ p / a [ 0 t ] / l 0 e ] l a c a n 0.3 a 0.4 n [ a / t a 0 ] u p e 0.9 b o d [ r y { p h [ n e l { d n l l
a t
0.2 e c
a 0.6 [ { n
l 0.2
0.1 0.3
ln{[but-1-ene] /{[but-1-ene] } ln{[propene] /[propene] } ln{[but-1-ene] /[but-1-ene] } 0 t 0 t 0 t 0.0 0.0 0.0 0.0 0.5 1.0 1.5 2.0 0.0 0.2 0.4 0.6 0.8 0.0 0.3 0.6 0.9 1.2 1.5 1.8
2.0 } ] t l
0.9 a } t n ] l } E a t D ]
a F l 1.6 p n a o a r n
1.6 x p a l t e y n h [ h e / t 0 p ] e [ l / a 0 ] m l n - a a 1.2 2 [ n x / a ]
e 0.6 0
1.2 l t h a n [ n e { a p n [ l p {
o
r n l p l
0.8 y
0.8 h t e m -
0.3 2 [ { n 0.4 l 0.4
ln{[but-1-ene] /[but-1-ene] } ln{[but-1-ene] /[but-1-ene] } ln{[but-1-ene] /[but-1-ene] } 0 t 0 t 0 t 0.0 0.0 0.0 0.0 0.3 0.6 0.9 1.2 1.5 0.0 0.4 0.8 1.2 1.6 0.0 0.3 0.6 0.9
1.6 } ] t l } ] t }
1.0 t a ] l l a n a a n n a p t G a t o u H r I u b 0.6 l p b l y l y h y t h h
0.8 e t
1.2 t e e M - m m i 2 - [ d / ] 3 0 - l [ / 2 a ] 0 , n l 2 a a 0.6 [ t / n ]
u 0.4 0 l a b t a l
u y n 0.8 h b a t l p e y o h r M t - p e l 2
0.4 [ y m { - h n t 3 l e [ {
m 0.2 i n
0.4 l d - 2 0.2 , 2 [ { n l ln{[but-1-ene] /[but-1-ene] } ln{[but-1-ene] /[but-1-ene] } ln{[but-1-ene] /[but-1-ene] } 0 t 0 t 0 t 0.0 0.0 0.0 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.0 0.2 0.4 0.6 0.8 0.0 0.1 0.2 0.3 0.4 0.5
} ] t
1.5 l a } t n } ] t ] l a l t a a u n n b a 0.9 a l t L t N
y 1.2 n M n e h e t p
1.2 p l e l y y m h i h t t d e e - m 3 m , - - 2 3 3 [ [ / / [ ] 0 0 /
0.9 l ] ] 0 l l
a 0.6 a 0.8 a n n n a t a a
t t u n n b e e l p y p l l h y y 0.6 t h e h t t e m e i m m d - - - 2 0.3 3
3 0.4 [ , [ { 3 { n [ l n
0.3 { l n l
ln{[but-1-ene] /[but-1-ene] } 0 t ln{[but-1-ene] /[but-1-ene] } ln{[but-1-ene] /[but-1-ene] } 0 t 0 t 0.0 0.0 0.0 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.0 0.2 0.4 0.6 0.0 0.2 0.4 0.6
1.6 } } t ] ] t 1.6 l l a a n n P a a O t t u n e b l
1.2 p l y y h t h e t -
e 1.2 2 [ m / - ] 0 l 4 a [ / n ] 0 l a a t
0.8 n u a b t l n 0.8 y e h t p l e - y 2 h [ t e { n m l - 4
0.4 [
{ 0.4 n l
ln{[but-1-ene] /[but-1-ene] } ln{[but-1-ene] /[but-1-ene] } 0 t 0 t 0.0 0.0 0.0 0.3 0.6 0.9 1.2 0.0 0.1 0.2 0.3 0.4 0.5 This journal is © The Owner Societies 2001
Figure S2.
0.8 } t ] } } ] t l t ] e 1.0 a l a d n a y n a p h
A t e o B u r d C l b p
0.9 [ a [ / / t 0 ] 0 ] e l l
c 0.8 a 0.6 a a n n [ a / a ] t 0 p e u o d b r [ y p { h [ e n { l d
0.6 n l l
a 0.6 t
0.4 e c a [ {
n
l 0.4
0.3 0.2 0.2
ln {[but-1-ene] /[but-1-ene] } ln{[propene] /[propene] } ln{[but-1-ene] /[but-1-ene] } 0 t 0 t 0 t 0.0 0.0 0.0 0.0 0.4 0.8 1.2 1.6 0.0 0.3 0.6 0.9 1.2 1.5 0.0 0.3 0.6 0.9 1.2
} ] t l
} 1.2 t a ] l n } t a ] a l n p a
a 0.8
0.9 o t F n E D r a n p e x l e p y / h 0 ] h [ l t / a 0 e ] l
n 0.9 a m a - t n 2 n a 0.6 [ e x / ] 0 l e p
0.6 [ a h { [ n { a n l p n l o
r
0.6 p l
0.4 y h t e
0.3 m - 2 [ { n
0.3 l 0.2
ln{[but-1-ene] /[but-1-ene] } ln{[but-1-ene] /[but-1-ene] } 0 t 0.0 ln{[but-1-ene] /[but-1-ene] } 0 t 0 t 0.0 0.0 0.0 0.3 0.6 0.9 1.2 0.0 0.3 0.6 0.9 1.2 0.0 0.2 0.4 0.6 0.8 1.0
1.6 } ] t } l ] t l ] } a t l a n a n a n a 1.2 t a p u 0.8 t o H b I G u l r b y p l l h y t y h e h 1.2 t t e m e - M m 3 - - [ / ] 2 2 0 l
0.6 [ [ / / ] a ] 0 0 l l n a
a 0.8 a n t n a u a t b p u l o 0.8 b y
r l h p y t l h e y
0.4 t h e m t - e M 3 - [ m - 2 { [ n 2 l
[ { 0.4
{ n l
n 0.4
l 0.2
ln{[but-1-ene] /[but-1-ene] } ln{[but-1-ene] /[but-1-ene] } 0 t ln{[but-1-ene] /[but-1-ene] } 0 t 0 t 0.0 0.0 0.0 0.0 0.2 0.4 0.6 0.8 1.0 0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 0.0 0.3 0.6 0.9 1.2 1.5
1.6 1.4 ] } t l } 0.8 ] t a l a n } ] t l a n t
1.4 a a t u n n b a 1.2 l t
M e
y N L n p l e h t y p e
1.2 l h t y m e h
0.6 i
t 1.0 d m e - - 3 m 3 ,
1.0 - [ / 3 ] 2 0 l [ [ / ] a / 0 l ] 0 l n a 0.8 a a n t n a n a t 0.8 t
e u
0.4 n p b l e l y p y
l 0.6 h h t y t e
e 0.6 h t m e m - i m 3 d [ - - {
2 0.4 3 , [ 0.4 n l 0.2 3 {
[ n l {
n l 0.2 0.2 ln{[but-1-ene] /[but-1-ene] } ln{[but-1-ene] /[but-1-ene] } ln{[but-1-ene] /[but-1-ene] } 0 t 0 t 0 t 0.0 0.0 0.0 0.0 0.2 0.4 0.6 0.8 1.0 1.2 0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4
1.2 1.4 } ] t } ] t l l a a n n a a t t u n 1.2 P b e O l p y l h y
0.9 t h e t - e
1.0 2 [ m / ] - 0 l 4 a [ / n ] 0 l a t a u n 0.8 a b t l n 0.6 y
e h t p l e - y 0.6 2 h [ t e { n m l
- 4 [ 0.4 {
0.3 n l
0.2 ln{[but-1-ene] /[but-1-ene] } ln{[but-1-ene] /[but-1-ene] } 0 t 0 t 0.0 0.0 0.0 0.2 0.4 0.6 0.8 1.0 1.2 0.0 0.2 0.4 0.6 0.8 1.0