Alapahoochee River Watershed R Watershed Restoration Action Strategy R November 2007 ( R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fish Consumption Guidelines: Rivers & Creeks
FRESHWATER FISH CONSUMPTION GUIDELINES: RIVERS & CREEKS NO RESTRICTIONS ONE MEAL PER WEEK ONE MEAL PER MONTH DO NOT EAT NO DATA Bass, LargemouthBass, Other Bass, Shoal Bass, Spotted Bass, Striped Bass, White Bass, Bluegill Bowfin Buffalo Bullhead Carp Catfish, Blue Catfish, Channel Catfish,Flathead Catfish, White Crappie StripedMullet, Perch, Yellow Chain Pickerel, Redbreast Redhorse Redear Sucker Green Sunfish, Sunfish, Other Brown Trout, Rainbow Trout, Alapaha River Alapahoochee River Allatoona Crk. (Cobb Co.) Altamaha River Altamaha River (below US Route 25) Apalachee River Beaver Crk. (Taylor Co.) Brier Crk. (Burke Co.) Canoochee River (Hwy 192 to Ogeechee River) Chattahoochee River (Helen to Lk. Lanier) (Buford Dam to Morgan Falls Dam) (Morgan Falls Dam to Peachtree Crk.) * (Peachtree Crk. to Pea Crk.) * (Pea Crk. to West Point Lk., below Franklin) * (West Point dam to I-85) (Oliver Dam to Upatoi Crk.) Chattooga River (NE Georgia, Rabun County) Chestatee River (below Tesnatee Riv.) Conasauga River (below Stateline) Coosa River (River Mile Zero to Hwy 100, Floyd Co.) Coosa River <32" (Hwy 100 to Stateline, Floyd Co.) >32" Coosa River (Coosa, Etowah below Thompson-Weinman dam, Oostanaula) Coosawattee River (below Carters) Etowah River (Dawson Co.) Etowah River (above Lake Allatoona) Etowah River (below Lake Allatoona dam) Flint River (Spalding/Fayette Cos.) Flint River (Meriwether/Upson/Pike Cos.) Flint River (Taylor Co.) Flint River (Macon/Dooly/Worth/Lee Cos.) <16" Flint River (Dougherty/Baker Mitchell Cos.) 16–30" >30" Gum Crk. (Crisp Co.) Holly Crk. (Murray Co.) Ichawaynochaway Crk. Kinchafoonee Crk. (above Albany) Little River (above Clarks Hill Lake) Little River (above Ga. Hwy 133, Valdosta) Mill Crk. -

List of TMDL Implementation Plans with Tmdls Organized by Basin
Latest 305(b)/303(d) List of Streams List of Stream Reaches With TMDLs and TMDL Implementation Plans - Updated June 2011 Total Maximum Daily Loadings TMDL TMDL PLAN DELIST BASIN NAME HUC10 REACH NAME LOCATION VIOLATIONS TMDL YEAR TMDL PLAN YEAR YEAR Altamaha 0307010601 Bullard Creek ~0.25 mi u/s Altamaha Road to Altamaha River Bio(sediment) TMDL 2007 09/30/2009 Altamaha 0307010601 Cobb Creek Oconee Creek to Altamaha River DO TMDL 2001 TMDL PLAN 08/31/2003 Altamaha 0307010601 Cobb Creek Oconee Creek to Altamaha River FC 2012 Altamaha 0307010601 Milligan Creek Uvalda to Altamaha River DO TMDL 2001 TMDL PLAN 08/31/2003 2006 Altamaha 0307010601 Milligan Creek Uvalda to Altamaha River FC TMDL 2001 TMDL PLAN 08/31/2003 Altamaha 0307010601 Oconee Creek Headwaters to Cobb Creek DO TMDL 2001 TMDL PLAN 08/31/2003 Altamaha 0307010601 Oconee Creek Headwaters to Cobb Creek FC TMDL 2001 TMDL PLAN 08/31/2003 Altamaha 0307010602 Ten Mile Creek Little Ten Mile Creek to Altamaha River Bio F 2012 Altamaha 0307010602 Ten Mile Creek Little Ten Mile Creek to Altamaha River DO TMDL 2001 TMDL PLAN 08/31/2003 Altamaha 0307010603 Beards Creek Spring Branch to Altamaha River Bio F 2012 Altamaha 0307010603 Five Mile Creek Headwaters to Altamaha River Bio(sediment) TMDL 2007 09/30/2009 Altamaha 0307010603 Goose Creek U/S Rd. S1922(Walton Griffis Rd.) to Little Goose Creek FC TMDL 2001 TMDL PLAN 08/31/2003 Altamaha 0307010603 Mushmelon Creek Headwaters to Delbos Bay Bio F 2012 Altamaha 0307010604 Altamaha River Confluence of Oconee and Ocmulgee Rivers to ITT Rayonier -
Tmdl Implementation Plan
STATE OF GEORGIA TMDL IMPLEMENTATION PLAN Prepared by The Georgia Department of Natural Resources Environmental Protection Division Atlanta, GA Introduction TMDL Implementation Plans are not required for the segments discussed below because Georgia Environmental Protection Division (Georgia EPD) has delisted them, United States Environmental Protection Agency (USEPA) has rescinded them, or the United States District Court for the Northern District Court of Georgia, Atlanta Division has vacated the order to establish them. Delisted Segments EPA requires Georgia EPD to demonstrate good cause for not including stream segment on the Section 303(d) list. Good cause includes, but is not limited to, more recent and accurate data, more sophisticated water quality modeling, flaws in the original analysis that led to the waterbody being listed or changes in conditions, e.g. new control equipment, or elimination of discharges. In its Draft 2002 Section 303(d) listing proposal Georgia EPD submitted good cause justification to EPA to delist the stream segments listed below from its 2002 Section 303(d) list. Georgia EPD delisted stream segments using methodology from the Water Quality in Georgia 1998- 99 Report (305(b) report), Appendix A, and USEPA document Guidance for 303(b) listing. In considering all the data on the affected segments EPD concluded that water quality is being met and removed them from the 2002 Section 303(d) list. Therefore, no TMDL Implementation Plans are required for the following delisted segments. Waterbody Name Stream Location River Basin Pollutant 1. Alapahoochee River Confluence of Mud and Grand Bay Cr. To Stateline Suwannee FCG(Hg) 2. Buck Creek Downstream Sylvania WPCP to Savannah River Savannah Cyanide, Zn, Tox 3. -

Guidelines for Eating Fish from Georgia Waters 2017
Guidelines For Eating Fish From Georgia Waters 2017 Georgia Department of Natural Resources 2 Martin Luther King, Jr. Drive, S.E., Suite 1252 Atlanta, Georgia 30334-9000 i ii For more information on fish consumption in Georgia, contact the Georgia Department of Natural Resources. Environmental Protection Division Watershed Protection Branch 2 Martin Luther King, Jr. Drive, S.E., Suite 1152 Atlanta, GA 30334-9000 (404) 463-1511 Wildlife Resources Division 2070 U.S. Hwy. 278, S.E. Social Circle, GA 30025 (770) 918-6406 Coastal Resources Division One Conservation Way Brunswick, Ga. 31520 (912) 264-7218 Check the DNR Web Site at: http://www.gadnr.org For this booklet: Go to Environmental Protection Division at www.gaepd.org, choose publications, then fish consumption guidelines. For the current Georgia 2015 Freshwater Sport Fishing Regulations, Click on Wild- life Resources Division. Click on Fishing. Choose Fishing Regulations. Or, go to http://www.gofishgeorgia.com For more information on Coastal Fisheries and 2015 Regulations, Click on Coastal Resources Division, or go to http://CoastalGaDNR.org For information on Household Hazardous Waste (HHW) source reduction, reuse options, proper disposal or recycling, go to Georgia Department of Community Affairs at http://www.dca.state.ga.us. Call the DNR Toll Free Tip Line at 1-800-241-4113 to report fish kills, spills, sewer over- flows, dumping or poaching (24 hours a day, seven days a week). Also, report Poaching, via e-mail using [email protected] Check USEPA and USFDA for Federal Guidance on Fish Consumption USEPA: http://www.epa.gov/ost/fishadvice USFDA: http://www.cfsan.fda.gov/seafood.1html Image Credits:Covers: Duane Raver Art Collection, courtesy of the U.S. -

Evaluation of the Surface-Water Data Network
EVALUATION OF THE SURFACE-WATER DATA NETWORK, SUWANNEE RIVER BASIN, FLORIDA, 1982 By Roger P. Rumenik and John E. Coffin U.S. GEOLOGICAL SURVEY WATER-RESOURCES INVESTIGATIONS REPORT 84-4245 Prepared in cooperation with the SUWANNEE RIVER WATER MANAGEMENT DISTRICT Tallahassee, Florida 1984 UNITED STATES DEPARTMENT OF THE INTERIOR WILLIAM P. CLARK, Secretary GEOLOGICAL SURVEY Dallas L. Peck, Director For additional information Copies of this report can write to: be purchased from: District Chief Open-File Services Section U.S. Geological Survey Western Distribution Branch Suite 3015 U.S. Geological Survey 227 North Bronough Street Box 25425, Federal Center Tallahassee, Florida 32301 Denver, Colorado 80225 (Telephone: (303) 236-7476) CONTENTS Page Abstract 1 Introduction 1 Background 3 Regional setting 3 Regional hydrologic system 4 Goals of the surface-water data network 5 Evaluation of the surface-water data network 10 Discussion of the evaluation 18 Conclusions 20 Selected references 21 ILLUSTRATIONS Page Figures 1.-5. Maps showing location of: 1. Area of investigation 2 2. Current-use stations 7 3. Long-term trend stations 8 4. Stations used in planning and design 9 5. Gaging stations in the Suwannee River basin 13 TABLES Page Table 1. Goals of the surface-water data network 6 2. Streamflow gaging stations in the Suwannee River basin 10 III CONVERSION FACTORS For those readers who may prefer to use International System units (SI) rather than inch-pound units published herein, the conversion factors for the terms used in this report are listed below: Multiply inch-pound units BZ To obtain SI units inch (in.) 25.4 millimeter (mm) foot (ft) 0.3048 meter (m) mile (mi) 1.609 kilometer (km) square mile (mi2 ) 2.590 square kilometer (km2 ) cubic foot per second 0.02832 cubic meter per second (ft3 /s) (m3 /s) IV EVALUATION OF THE SURFACE-WATER DATA NETWORK, SUWANNEE RIVER BASIN, FLORIDA, 1982 By Roger P. -

Alapaha Riverand Tim Suwannee Rtver from Ti{E Illegal Discharge of Raw Sewage by Tiie City of Valdosta
RESOLUTTON 2or7 -ol ARESOLUTION BY TIIE BOARD OF COUNTY COMMISSIONERS OF HAMILTON COUNTY, FLORIDA DEMANDING PROTECTION OF TIIE WITIILACOOCI{EE RTVER THE ALAPAHOOCHEE RIVER. TIM ALAPAHA RIVERAND TIM SUWANNEE RTVER FROM TI{E ILLEGAL DISCHARGE OF RAW SEWAGE BY TIIE CITY OF VALDOSTA. GEORGIA. WHEREAS, the Withlacoochee River forms the Western boundary of Hamilton County, Florida from the Florida/Georgia state line until its juncture with the Suwannee River; and WHEREAS, the Withlacoochee River provides recreational and economic benefits to the citizens of Hamilton County and others; and WHEREAS, citizens of Hamilton County depend on clean water from the Withlacoochee River watershed for activities of daily life as well as for agricultural, business, and recreational purposes; and WHEREAS, the health and welfare of many Hamilton County citizens is directly harmed by any degradation of the quality of water in the Withlacoochee River and its watershed; and WHEREAS, the City of Valdosta, Georgia over the past several years has experienced numerous failures and spills with their wastewater treatment facility; which resulted in the construction of a new facility that was placed into service in 2016; and WHEREAS, the City of Valdosta, on January 22,2017, had yet another failure which resulted in the release of 2.2 million gallons of raw sewage and stormwater into the Wthlacoochee River; and WHEREAS, the Hamilton County Health Department has issued numerous health advisories warning of the dangers of contact with the waters contaminated by sewage -

Streamflow Maps of Georgia's Major Rivers
GEORGIA STATE DIVISION OF CONSERVATION DEPARTMENT OF MINES, MINING AND GEOLOGY GARLAND PEYTON, Director THE GEOLOGICAL SURVEY Information Circular 21 STREAMFLOW MAPS OF GEORGIA'S MAJOR RIVERS by M. T. Thomson United States Geological Survey Prepared cooperatively by the Geological Survey, United States Department of the Interior, Washington, D. C. ATLANTA 1960 STREAMFLOW MAPS OF GEORGIA'S MAJOR RIVERS by M. T. Thomson Maps are commonly used to show the approximate rates of flow at all localities along the river systems. In addition to average flow, this collection of streamflow maps of Georgia's major rivers shows features such as low flows, flood flows, storage requirements, water power, the effects of storage reservoirs and power operations, and some comparisons of streamflows in different parts of the State. Most of the information shown on the streamflow maps was taken from "The Availability and use of Water in Georgia" by M. T. Thomson, S. M. Herrick, Eugene Brown, and others pub lished as Bulletin No. 65 in December 1956 by the Georgia Department of Mines, Mining and Geo logy. The average flows reported in that publication and sho\vn on these maps were for the years 1937-1955. That publication should be consulted for detailed information. More recent streamflow information may be obtained from the Atlanta District Office of the Surface Water Branch, Water Resources Division, U. S. Geological Survey, 805 Peachtree Street, N.E., Room 609, Atlanta 8, Georgia. In order to show the streamflows and other features clearly, the river locations are distorted slightly, their lengths are not to scale, and some features are shown by block-like patterns. -

Alapaha River Mercury TMDL Report
Total Maximum Daily Load for Total Mercury in the Alapaha Watershed, GA February 28, 2002 TOTAL MAXIMUM DAILY LOAD (TMDL) DEVELOPMENT For Total Mercury in the Alapaha Watershed Including Listed Segments of the Alapaha River: Alapaha River: Sand Creek to US Highway 129 Alapaha River: US Highway 129/GA Highway 11 to Stateline Double Run Creek Alapahoochee River Total Maximum Daily Load for Total Mercury in the Alapaha Watershed, GA February 28, 2002 TOTAL MAXIMUM DAILY LOAD (TMDL) Total Mercury in Fish Tissue Residue In the In the Alapaha River Watershed Under the authority of Section 303(d) of the Clean Water Act, 33 U.S.C. 1251 et seq., as amended by the Water Quality Act of 1987, P.L. 100-4, the U.S. Environmental Protection Agency is hereby establishing a TMDL for total mercury for the protection of public health associated with the consumption of fish taken from the following segments of the Alapaha River in Georgia: Alapaha River: Sand Creek to US Highway 129 Alapaha River: US Highway 129/GA Highway 11 to Stateline Double Run Creek Alapahoochee River The calculated allowable load of mercury that may come into the identified segments of the Alapaha River without exceeding the applicable water quality standard is 2.62 kilograms per year. The applicable water quality standard is the State of Georgia’s numeric interpretation of their narrative water quality standard for protection of human health from toxic substances. This interpretation indicates that the consumption of fish by the general population is not to exceed 0.3 mg/kg mercury in fish tissue. -
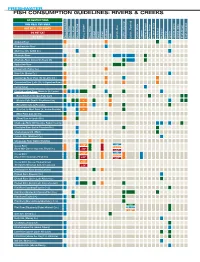
Fish Consumption Guidelines: Rivers & Creeks
FRESHWATER FISH CONSUMPTION GUIDELINES: RIVERS & CREEKS NO RESTRICTIONS ONE MEAL PER WEEK ONE MEAL PER MONTH DO NOT EAT NO DATA Bass, LargemouthBass, Other Bass, Shoal Bass, Spotted Bass, Striped Bass, White Bass, Bluegill Bowfin Buffalo Bullhead Carp Catfish, Blue Catfish, Channel Catfish,Flathead Catfish, White Crappie StripedMullet, Perch, Yellow Chain Pickerel, Redbreast Redhorse Redear Sucker Green Sunfish, Sunfish, Other Brown Trout, Rainbow Trout, Alapaha River Alapahoochee River Allatoona Crk. (Cobb Co.) Altamaha River Altamaha River (below US Route 25) Apalachee River Beaver Crk. (Taylor Co.) Brier Crk. (Burke Co.) Canoochee River (Hwy 192 to Lotts Crk.) Canoochee River (Lotts Crk. to Ogeechee River) Casey Canal Chattahoochee River (Helen to Lk. Lanier) (Buford Dam to Morgan Falls Dam) (Morgan Falls Dam to Peachtree Crk.) * (Peachtree Crk. to Pea Crk.) * (Pea Crk. to West Point Lk., below Franklin) * (West Point dam to I-85) (Oliver Dam to Upatoi Crk.) Chattooga River (NE Georgia, Rabun County) Chestatee River (below Tesnatee Riv.) Chickamauga Crk. (West) Cohulla Crk. (Whitfield Co.) Conasauga River (below Stateline) <18" Coosa River <20" 18 –32" (River Mile Zero to Hwy 100, Floyd Co.) ≥20" >32" <18" Coosa River <20" 18 –32" (Hwy 100 to Stateline, Floyd Co.) ≥20" >32" Coosa River (Coosa, Etowah below <20" Thompson-Weinman dam, Oostanaula) ≥20" Coosawattee River (below Carters) Etowah River (Dawson Co.) Etowah River (above Lake Allatoona) Etowah River (below Lake Allatoona dam) Flint River (Spalding/Fayette Cos.) Flint River (Meriwether/Upson/Pike Cos.) Flint River (Taylor Co.) Flint River (Macon/Dooly/Worth/Lee Cos.) <16" Flint River (Dougherty/Baker Mitchell Cos.) 16–30" >30" Gum Crk. -

2011 Regionally Important Resources Plan Regionally Important Resources 2011 1
2011 Regionally Important Resources Plan Regionally Important Resources 2011 1 REGIONALLY IMPORTANT RESOURCES PLAN Southern Georgia July 2011 Prepared by: Valdosta Office 327 West Savannah Avenue Valdosta, GA 31601 Phone: 229.333.5277 Fax: 229.333.5312 Waycross Office 1725 S. GA Parkway, W. Waycross, GA 31503 Phone: 912.285.6097 Fax: 912.285.6126 2 TABLE OF CONTENTS Executive Summary The Purpose The Process The Plan Identification of Resources Introduction Background Designation of Regionally Important Resources Methodology and Process Nomination and Evaluation Research and Data Collection Criteria for Determining Value of RIR Identification of Vulnerability of RIR Stakeholder Review Regionally Important Resources Map Value Matrix Vulnerability Matrix Resource Narrative: Historic & Cultural Resources Resource Narrative: Areas of Conservation & Recreational Value Appendices Appendix A: Stakeholder List Appendix B: Regionally Important Resources Nomination Form Appendix C: List of Regionally Important Resources Appendix D: Regional Resource Plan Briefings and Presentations Appendix E: References 3 Regionally Important Resources Plan EXECUTIVE SUMMARY THE PURPOSE Pursuant to Rules of the Department of Community Affairs, Chapter 110-12-4, Regionally Important Resources are defined as “Any natural or cultural resource area identified for protection by a Regional Commission following the minimum requirements established by the Department.” The Regional Resource Plan is designed to: Enhance the focus on protection and management of important natural and cultural resources in the Southern Georgia Region. Provide for careful consideration of, and planning for, impacts of new development on these important resources. Improve local, regional, and state level coordination in the protection and management of identified resources. THE PROCESS The public nomination process resulted in twenty—one nominations from local governments, non-profit agencies, and private citizens. -

Class G Tables of Geographic Cutter Numbers: Maps -- by Region Or
G3862 SOUTHERN STATES. REGIONS, NATURAL G3862 FEATURES, ETC. .C55 Clayton Aquifer .C6 Coasts .E8 Eutaw Aquifer .G8 Gulf Intracoastal Waterway .L6 Louisville and Nashville Railroad 525 G3867 SOUTHEASTERN STATES. REGIONS, NATURAL G3867 FEATURES, ETC. .C5 Chattahoochee River .C8 Cumberland Gap National Historical Park .C85 Cumberland Mountains .F55 Floridan Aquifer .G8 Gulf Islands National Seashore .H5 Hiwassee River .J4 Jefferson National Forest .L5 Little Tennessee River .O8 Overmountain Victory National Historic Trail 526 G3872 SOUTHEAST ATLANTIC STATES. REGIONS, G3872 NATURAL FEATURES, ETC. .B6 Blue Ridge Mountains .C5 Chattooga River .C52 Chattooga River [wild & scenic river] .C6 Coasts .E4 Ellicott Rock Wilderness Area .N4 New River .S3 Sandhills 527 G3882 VIRGINIA. REGIONS, NATURAL FEATURES, ETC. G3882 .A3 Accotink, Lake .A43 Alexanders Island .A44 Alexandria Canal .A46 Amelia Wildlife Management Area .A5 Anna, Lake .A62 Appomattox River .A64 Arlington Boulevard .A66 Arlington Estate .A68 Arlington House, the Robert E. Lee Memorial .A7 Arlington National Cemetery .A8 Ash-Lawn Highland .A85 Assawoman Island .A89 Asylum Creek .B3 Back Bay [VA & NC] .B33 Back Bay National Wildlife Refuge .B35 Baker Island .B37 Barbours Creek Wilderness .B38 Barboursville Basin [geologic basin] .B39 Barcroft, Lake .B395 Battery Cove .B4 Beach Creek .B43 Bear Creek Lake State Park .B44 Beech Forest .B454 Belle Isle [Lancaster County] .B455 Belle Isle [Richmond] .B458 Berkeley Island .B46 Berkeley Plantation .B53 Big Bethel Reservoir .B542 Big Island [Amherst County] .B543 Big Island [Bedford County] .B544 Big Island [Fluvanna County] .B545 Big Island [Gloucester County] .B547 Big Island [New Kent County] .B548 Big Island [Virginia Beach] .B55 Blackwater River .B56 Bluestone River [VA & WV] .B57 Bolling Island .B6 Booker T. -

Final Progress Report ARWT 2015-05-15
5/15/2015 https://sites.google.com/site/wwalswatersheds/alapaha-water-trail/final-progress-report-arwt-2015-05-15?tmpl=%2Fsystem%2Fapp%2Ftemplates%2Fprint%2F&showPrin… Alapaha River Water Trail > Final Progress Report ARWT 2015-05-15 From: John S. Quarterman, President, WWALS Watershed Coalition, Inc. To: Dana Skelton, Director of Administration & Outreach, Georgia River Network Cc: Gwyneth Moody, Water Trails Coordinator, Georgia River Network As requested in the grant contract, here is the final progress report for the Alapaha River Water Trail (ARWT). Summary: WWALS has spent the entire $500 grant amount on purchasing marketing materials to promote the project and encourage community use of the river. WWALS also raised an additional $750 from other sponsors, and spent most of those funds similarly; the remainder is for signs at access points. We have met all the requirements of the grant contract, and we have already met all the stated criteria of a Water Trail except placing signage at access points. The members of the Alapaha River Water Trail Committee and WWALS continue to do more. Unusual features of the ARWT include: the Alapaha River runs between two states (Georgia and Florida) with different legal and landowner situations. The Alapaha River Basin includes several tributary rivers: the Willacoochee River, Alapahoochee River, Dead River, and the Little Alapaha River, the last three of which and the Alapaha River itself at least sometimes disappear into sinks at the Cody Scarp, a geological feature of north Florida that so far as we know has no parallel in Georgia. Water levels in all these rivers vary widely during the year, depending on rains, and WWALS publishes a web page with water levels at all the gauges on the Alapaha River, plus a table of boatable low and safe high levels at each gauge appears there and on the printed materials.